Alkali Metals
| primordial |
| element by radioactive decay |
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element.
The alkali metals are all shiny, soft, highly reactive metals at standard temperature and pressure and readily lose their outermost electron to form cations with charge +1. They can all be cut easily with a knife due to their softness, exposing a shiny surface that tarnishes rapidly in air due to oxidation by atmospheric moisture and oxygen (and in the case of lithium, nitrogen). Because of their high reactivity, they must be stored under oil to prevent reaction with air, and are found naturally only in salts and never as the free elements. Caesium, the fifth alkali metal, is the most reactive of all the metals. All the alkali metals react with water, with the heavier alkali metals reacting more vigorously than the lighter ones.
All of the discovered alkali metals occur in nature as their compounds: in order of abundance, sodium is the most abundant, followed by potassium, lithium, rubidium, caesium, and finally francium, which is very rare due to its extremely high radioactivity; francium occurs only in minute traces in nature as an intermediate step in some obscure side branches of the natural decay chains. Experiments have been conducted to attempt the synthesis of element 119, which is likely to be the next member of the group; none were successful. However, ununennium may not be an alkali metal due to relativistic effects, which are predicted to have a large influence on the chemical properties of superheavy elements; even if it does turn out to be an alkali metal, it is predicted to have some differences in physical and chemical properties from its lighter homologues.
Most alkali metals have many different applications. One of the best-known applications of the pure elements is the use of rubidium and caesium in atomic clocks, of which caesium atomic clocks form the basis of the second. A common application of the compounds of sodium is the sodium-vapour lamp, which emits light very efficiently. Table salt, or sodium chloride, has been used since antiquity. Lithium finds use as a psychiatric medication and as an anode in lithium batteries. Sodium, potassium and possibly lithium are essential elements, having major biological roles as electrolytes, and although the other alkali metals are not essential, they also have various effects on the body, both beneficial and harmful.
History

Sodium compounds have been known since ancient times; salt (sodium chloride) has been an important commodity in human activities. While potash has been used since ancient times, it was not understood for most of its history to be a fundamentally different substance from sodium mineral salts. Georg Ernst Stahl obtained experimental evidence which led him to suggest the fundamental difference of sodium and potassium salts in 1702, and Henri-Louis Duhamel du Monceau was able to prove this difference in 1736. The exact chemical composition of potassium and sodium compounds, and the status as chemical element of potassium and sodium, was not known then, and thus Antoine Lavoisier did not include either alkali in his list of chemical elements in 1789.
Pure potassium was first isolated in 1807 in England by Humphry Davy, who derived it from caustic potash (KOH, potassium hydroxide) by the use of electrolysis of the molten salt with the newly invented voltaic pile. Previous attempts at electrolysis of the aqueous salt were unsuccessful due to potassium's extreme reactivity. Potassium was the first metal that was isolated by electrolysis. Later that same year, Davy reported extraction of sodium from the similar substance caustic soda (NaOH, lye) by a similar technique, demonstrating the elements, and thus the salts, to be different.

Petalite (LiAlSi4O10) was discovered in 1800 by the Brazilian chemist José Bonifácio de Andrada in a mine on the island of Utö, Sweden. However, it was not until 1817 that Johan August Arfwedson, then working in the laboratory of the chemist Jöns Jacob Berzelius, detected the presence of a new element while analysing petalite ore. This new element was noted by him to form compounds similar to those of sodium and potassium, though its carbonate and hydroxide were less soluble in water and more alkaline than the other alkali metals. Berzelius gave the unknown material the name lithion/lithina, from the Greek word λιθoς (transliterated as lithos, meaning "stone"), to reflect its discovery in a solid mineral, as opposed to potassium, which had been discovered in plant ashes, and sodium, which was known partly for its high abundance in animal blood. He named the metal inside the material lithium. Lithium, sodium, and potassium were part of the discovery of periodicity, as they are among a series of triads of elements in the same group that were noted by Johann Wolfgang Döbereiner in 1850 as having similar properties.

Rubidium and caesium were the first elements to be discovered using the spectroscope, invented in 1859 by Robert Bunsen and Gustav Kirchhoff. The next year, they discovered caesium in the mineral water from Bad Dürkheim, Germany. Their discovery of rubidium came the following year in Heidelberg, Germany, finding it in the mineral lepidolite. The names of rubidium and caesium come from the most prominent lines in their emission spectra: a bright red line for rubidium (from the Latin word rubidus, meaning dark red or bright red), and a sky-blue line for caesium (derived from the Latin word caesius, meaning sky-blue).
Around 1865 John Newlands produced a series of papers where he listed the elements in order of increasing atomic weight and similar physical and chemical properties that recurred at intervals of eight; he likened such periodicity to the octaves of music, where notes an octave apart have similar musical functions. His version put all the alkali metals then known (lithium to caesium), as well as copper, silver, and thallium (which show the +1 oxidation state characteristic of the alkali metals), together into a group. His table placed hydrogen with the halogens.
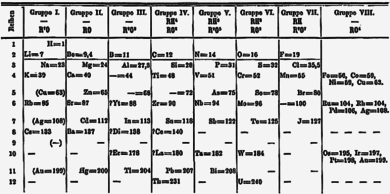
After 1869, Dmitri Mendeleev proposed his periodic table placing lithium at the top of a group with sodium, potassium, rubidium, caesium, and thallium. Two years later, Mendeleev revised his table, placing hydrogen in group 1 above lithium, and also moving thallium to the boron group. In this 1871 version, copper, silver, and gold were placed twice, once as part of group IB, and once as part of a "group VIII" encompassing today's groups 8 to 11. After the introduction of the 18-column table, the group IB elements were moved to their current position in the d-block, while alkali metals were left in group IA. Later the group's name was changed to group 1 in 1988. The trivial name "alkali metals" comes from the fact that the hydroxides of the group 1 elements are all strong alkalis when dissolved in water.
There were at least four erroneous and incomplete discoveries before Marguerite Perey of the Curie Institute in Paris, France discovered francium in 1939 by purifying a sample of actinium-227, which had been reported to have a decay energy of 220 keV. However, Perey noticed decay particles with an energy level below 80 keV. Perey thought this decay activity might have been caused by a previously unidentified decay product, one that was separated during purification, but emerged again out of the pure actinium-227. Various tests eliminated the possibility of the unknown element being thorium, radium, lead, bismuth, or thallium. The new product exhibited chemical properties of an alkali metal (such as coprecipitating with caesium salts), which led Perey to believe that it was element 87, caused by the alpha decay of actinium-227. Perey then attempted to determine the proportion of beta decay to alpha decay in actinium-227. Her first test put the alpha branching at 0.6%, a figure that she later revised to 1%.
89Ac
87Fr
88Ra
86Rn
The next element below francium (eka-francium) in the periodic table would be ununennium (Uue), element 119. The synthesis of ununennium was first attempted in 1985 by bombarding a target of einsteinium-254 with calcium-48 ions at the superHILAC accelerator at the Lawrence Berkeley National Laboratory in Berkeley, California. No atoms were identified, leading to a limiting yield of 300 nb.
It is highly unlikely that this reaction will be able to create any atoms of ununennium in the near future, given the extremely difficult task of making sufficient amounts of einsteinium-254, which is favoured for production of ultraheavy elements because of its large mass, relatively long half-life of 270 days, and availability in significant amounts of several micrograms, to make a large enough target to increase the sensitivity of the experiment to the required level; einsteinium has not been found in nature and has only been produced in laboratories, and in quantities smaller than those needed for effective synthesis of superheavy elements. However, given that ununennium is only the first period 8 element on the extended periodic table, it may well be discovered in the near future through other reactions, and indeed an attempt to synthesise it is currently ongoing in Japan. Currently, none of the period 8 elements has been discovered yet, and it is also possible, due to drip instabilities, that only the lower period 8 elements, up to around element 128, are physically possible. No attempts at synthesis have been made for any heavier alkali metals: due to their extremely high atomic number, they would require new, more powerful methods and technology to make.
Occurrence
In the Solar System

The Oddo–Harkins rule holds that elements with even atomic numbers are more common that those with odd atomic numbers, with the exception of hydrogen. This rule argues that elements with odd atomic numbers have one unpaired proton and are more likely to capture another, thus increasing their atomic number. In elements with even atomic numbers, protons are paired, with each member of the pair offsetting the spin of the other, enhancing stability. All the alkali metals have odd atomic numbers and they are not as common as the elements with even atomic numbers adjacent to them (the noble gases and the alkaline earth metals) in the Solar System. The heavier alkali metals are also less abundant than the lighter ones as the alkali metals from rubidium onward can only be synthesised in supernovae and not in stellar nucleosynthesis. Lithium is also much less abundant than sodium and potassium as it is poorly synthesised in both Big Bang nucleosynthesis and in stars: the Big Bang could only produce trace quantities of lithium, beryllium and boron due to the absence of a stable nucleus with 5 or 8 nucleons, and stellar nucleosynthesis could only pass this bottleneck by the triple-alpha process, fusing three helium nuclei to form carbon, and skipping over those three elements.
On Earth

The Earth formed from the same cloud of matter that formed the Sun, but the planets acquired different compositions during the formation and evolution of the solar system. In turn, the natural history of the Earth caused parts of this planet to have differing concentrations of the elements. The mass of the Earth is approximately 5.98×10 kg. It is composed mostly of iron (32.1%), oxygen (30.1%), silicon (15.1%), magnesium (13.9%), sulfur (2.9%), nickel (1.8%), calcium (1.5%), and aluminium (1.4%); with the remaining 1.2% consisting of trace amounts of other elements. Due to planetary differentiation, the core region is believed to be primarily composed of iron (88.8%), with smaller amounts of nickel (5.8%), sulfur (4.5%), and less than 1% trace elements.
The alkali metals, due to their high reactivity, do not occur naturally in pure form in nature. They are lithophiles and therefore remain close to the Earth's surface because they combine readily with oxygen and so associate strongly with silica, forming relatively low-density minerals that do not sink down into the Earth's core. Potassium, rubidium and caesium are also incompatible elements due to their large ionic radii.
Sodium and potassium are very abundant on Earth, both being among the ten most common elements in Earth's crust; sodium makes up approximately 2.6% of the Earth's crust measured by weight, making it the sixth most abundant element overall and the most abundant alkali metal. Potassium makes up approximately 1.5% of the Earth's crust and is the seventh most abundant element. Sodium is found in many different minerals, of which the most common is ordinary salt (sodium chloride), which occurs in vast quantities dissolved in seawater. Other solid deposits include halite, amphibole, cryolite, nitratine, and zeolite. Many of these solid deposits occur as a result of ancient seas evaporating, which still occurs now in places such as Utah's Great Salt Lake and the Dead Sea. Despite their near-equal abundance in Earth's crust, sodium is far more common than potassium in the ocean, both because potassium's larger size makes its salts less soluble, and because potassium is bound by silicates in soil and what potassium leaches is absorbed far more readily by plant life than sodium.
Despite its chemical similarity, lithium typically does not occur together with sodium or potassium due to its smaller size. Due to its relatively low reactivity, it can be found in seawater in large amounts; it is estimated that lithium concentration in seawater is approximately 0.14 to 0.25 parts per million (ppm) or 25 micromolar. Its diagonal relationship with magnesium often allows it to replace magnesium in ferromagnesium minerals, where its crustal concentration is about 18 ppm, comparable to that of gallium and niobium. Commercially, the most important lithium mineral is spodumene, which occurs in large deposits worldwide.
Rubidium is approximately as abundant as zinc and more abundant than copper. It occurs naturally in the minerals leucite, pollucite, carnallite, zinnwaldite, and lepidolite, although none of these contain only rubidium and no other alkali metals. Caesium is more abundant than some commonly known elements, such as antimony, cadmium, tin, and tungsten, but is much less abundant than rubidium.
Francium-223, the only naturally occurring isotope of francium, is the product of the alpha decay of actinium-227 and can be found in trace amounts in uranium minerals. In a given sample of uranium, there is estimated to be only one francium atom for every 10 uranium atoms. It has been calculated that there are at most 30 grams of francium in the earth's crust at any time, due to its extremely short half-life of 22 minutes.
Properties
Physical and chemical
The physical and chemical properties of the alkali metals can be readily explained by their having an ns valence electron configuration, which results in weak metallic bonding. Hence, all the alkali metals are soft and have low densities, melting and boiling points, as well as heats of sublimation, vaporisation, and dissociation. They all crystallise in the body-centered cubic crystal structure, and have distinctive flame colours because their outer s electron is very easily excited. Indeed, these flame test colours are the most common way of identifying them since all their salts with common ions are soluble. The ns configuration also results in the alkali metals having very large atomic and ionic radii, as well as very high thermal and electrical conductivity. Their chemistry is dominated by the loss of their lone valence electron in the outermost s-orbital to form the +1 oxidation state, due to the ease of ionising this electron and the very high second ionisation energy. Most of the chemistry has been observed only for the first five members of the group. The chemistry of francium is not well established due to its extreme radioactivity; thus, the presentation of its properties here is limited. What little is known about francium shows that it is very close in behaviour to caesium, as expected. The physical properties of francium are even sketchier because the bulk element has never been observed; hence any data that may be found in the literature are certainly speculative extrapolations.
| Name | Lithium | Sodium | Potassium | Rubidium | Caesium | Francium |
|---|---|---|---|---|---|---|
| Atomic number | 3 | 11 | 19 | 37 | 55 | 87 |
| Standard atomic weight | 6.94(1) | 22.98976928(2) | 39.0983(1) | 85.4678(3) | 132.9054519(2) | [223] |
| Electron configuration | [He] 2s | [Ne] 3s | [Ar] 4s | [Kr] 5s | [Xe] 6s | [Rn] 7s |
| Melting point (°C) | 180.54 | 97.72 | 63.38 | 39.31 | 28.44 | ? |
| Boiling point (°C) | 1342 | 883 | 759 | 688 | 671 | ? |
| Density (g·cm) | 0.534 | 0.968 | 0.89 | 1.532 | 1.93 | ? |
| Heat of fusion (kJ·mol) | 3.00 | 2.60 | 2.321 | 2.19 | 2.09 | ? |
| Heat of vaporisation (kJ·mol) | 136 | 97.42 | 79.1 | 69 | 66.1 | ? |
| Heat of formation of monatomic gas (kJ·mol) | 162 | 108 | 89.6 | 82.0 | 78.2 | ? |
| Electrical resistivity at 25 °C (nΩ·cm) | 94.7 | 48.8 | 73.9 | 131 | 208 | ? |
| Atomic radius (pm) | 152 | 186 | 227 | 248 | 265 | ? |
| Ionic radius of hexacoordinate M ion (pm) | 76 | 102 | 138 | 152 | 167 | ? |
| First ionisation energy (kJ·mol) | 520.2 | 495.8 | 418.8 | 403.0 | 375.7 | 392.8 |
| Electron affinity (kJ·mol) | 59.62 | 52.87 | 48.38 | 46.89 | 45.51 | ? |
| Enthalpy of dissociation of M2 (kJ·mol) | 106.5 | 73.6 | 57.3 | 45.6 | 44.77 | ? |
| Pauling electronegativity | 0.98 | 0.93 | 0.82 | 0.82 | 0.79 | ? |
| Allen electronegativity | 0.91 | 0.87 | 0.73 | 0.71 | 0.66 | 0.67 |
| Standard electrode potential (E°(M→M); V) | −3.04 | −2.71 | −2.93 | −2.98 | −3.03 | ? |
| Flame test colour Principal emission/absorption wavelength (nm) |
Crimson 670.8 |
Yellow 589.2 |
Violet 766.5 |
Red-violet 780.0 |
Blue 455.5 |
? |
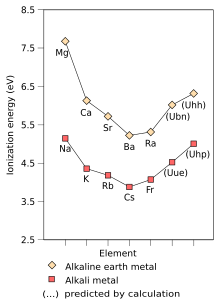
The alkali metals are more similar to each other than the elements in any other group are to each other. Indeed, the similarity is so great that it is quite difficult to separate potassium, rubidium, and caesium, due to their similar ionic radii; lithium and sodium are more distinct. For instance, when moving down the table, all known alkali metals show increasing atomic radius, decreasing electronegativity, increasing reactivity, and decreasing melting and boiling points as well as heats of fusion and vaporisation. In general, their densities increase when moving down the table, with the exception that potassium is less dense than sodium. One of the very few properties of the alkali metals that does not display a very smooth trend is their reduction potentials: lithium's value is anomalous, being more negative than the others. This is because the Li ion has a very high hydration energy in the gas phase: though the lithium ion disrupts the structure of water significantly, causing a higher change in entropy, this high hydration energy is enough to make the reduction potentials indicate it as being the most electropositive alkali metal, despite the difficulty of ionising it in the gas phase.
The stable alkali metals are all silver-coloured metals except for caesium, which has a pale golden tint: it is one of only three metals that are clearly coloured (the other two being copper and gold). Additionally, the heavy alkaline earth metals calcium, strontium, and barium, as well as the divalent lanthanides europium and ytterbium, are pale yellow, though the colour is much less prominent than it is for caesium. Their lustre tarnishes rapidly in air due to oxidation.
All the alkali metals are highly reactive and are never found in elemental forms in nature. Because of this, they are usually stored in mineral oil or kerosene (paraffin oil). They react aggressively with the halogens to form the alkali metal halides, which are white ionic crystalline compounds that are all soluble in water except lithium fluoride (LiF). The alkali metals also react with water to form strongly alkaline hydroxides and thus should be handled with great care. The heavier alkali metals react more vigorously than the lighter ones; for example, when dropped into water, caesium produces a larger explosion than potassium if the same number of moles of each metal is used. The alkali metals have the lowest first ionisation energies in their respective periods of the periodic table because of their low effective nuclear charge and the ability to attain a noble gas configuration by losing just one electron. Not only do the alkali metals react with water, but also with proton donors like alcohols and phenols, gaseous ammonia, and alkynes, the last demonstrating the phenomenal degree of their reactivity. Their great power as reducing agents makes them very useful in liberating other metals from their oxides or halides.
The second ionisation energy of all of the alkali metals is very high as it is in a full shell that is also closer to the nucleus; thus, they almost always lose a single electron, forming cations. The alkalides are an exception: they are unstable compounds which contain alkali metals in a −1 oxidation state, which is very unusual as before the discovery of the alkalides, the alkali metals were not expected to be able to form anions and were thought to be able to appear in salts only as cations. The alkalide anions have filled s-subshells, which gives them enough stability to exist. All the stable alkali metals except lithium are known to be able to form alkalides, and the alkalides have much theoretical interest due to their unusual stoichiometry and low ionisation potentials. Alkalides are chemically similar to the electrides, which are salts with trapped electrons acting as anions. A particularly striking example of an alkalide is "inverse sodium hydride", HNa (both ions being complexed), as opposed to the usual sodium hydride, NaH: it is unstable in isolation, due to its high energy resulting from the displacement of two electrons from hydrogen to sodium, although several derivatives are predicted to be metastable or stable.
In aqueous solution, the alkali metal ions form aqua ions of the formula [M(H2O)n], where n is the solvation number. Their coordination numbers and shapes agree well with those expected from their ionic radii. In aqueous solution the water molecules directly attached to the metal ion are said to belong to the first coordination sphere, also known as the first, or primary, solvation shell. The bond between a water molecule and the metal ion is a dative covalent bond, with the oxygen atom donating both electrons to the bond. Each coordinated water molecule may be attached by hydrogen bonds to other water molecules. The latter are said to reside in the second coordination sphere. However, for the alkali metal cations, the second coordination sphere is not well-defined as the +1 charge on the cation is not high enough to polarise the water molecules in the primary solvation shell enough for them to form strong hydrogen bonds with those in the second coordination sphere, producing a more stable entity. The solvation number for Li has been experimentally determined to be 4, forming the tetrahedral [Li(H2O)4]: while solvation numbers of 3 to 6 have been found for lithium aqua ions, solvation numbers less than 4 may be the result of the formation of contact ion pairs, and the higher solvation numbers may be interpreted in terms of water molecules that approach [Li(H2O)4] through a face of the tetrahedron, though molecular dynamic simulations may indicate the existence of an octahedral hexaaqua ion. There are also probably six water molecules in the primary solvation sphere of the sodium ion, forming the octahedral [Na(H2O)6] ion. While it was previously thought that the heavier alkali metals also formed octahedral hexaaqua ions, it has since been found that potassium and rubidium probably form the [K(H2O)8] and [Rb(H2O)8] ions, which have the square antiprismatic structure, and that caesium forms the 12-coordinate [Cs(H2O)12] ion.
Lithium
The chemistry of lithium shows several differences from that of the rest of the group as the small Li cation polarises anions and gives its compounds a more covalent character. Lithium and magnesium have a diagonal relationship due to their similar atomic radii, so that they show some similarities. For example, lithium forms a stable nitride, a property common among all the alkaline earth metals (magnesium's group) but unique among the alkali metals. In addition, among their respective groups, only lithium and magnesium form organometallic compounds with significant covalent character (e.g. LiMe and MgMe2).
Lithium fluoride is the only alkali metal halide that is poorly soluble in water, and lithium hydroxide is the only alkali metal hydroxide that is not deliquescent. Conversely, lithium perchlorate and other lithium salts with large anions that cannot be polarised are much more stable than the analogous compounds of the other alkali metals, probably because Li has a high solvation energy. This effect also means that most simple lithium salts are commonly encountered in hydrated form, because the anhydrous forms are extremely hygroscopic: this allows salts like lithium chloride and lithium bromide to be used in dehumidifiers and air-conditioners.
Francium
Francium is also predicted to show some differences due to its high atomic weight, causing its electrons to travel at considerable fractions of the speed of light and thus making relativistic effects more prominent. In contrast to the trend of decreasing electronegativities and ionisation energies of the alkali metals, francium's electronegativity and ionisation energy are predicted to be higher than caesium's due to the relativistic stabilisation of the 7s electrons; also, its atomic radius is expected to be abnormally low. Thus, contrary to expectation, caesium is the most reactive of the alkali metals, not francium. All known physical properties of francium also deviate from the clear trends going from lithium to caesium, such as the first ionisation energy, electron affinity, and anion polarisability, though due to the paucity of known data about francium many sources give extrapolated values, ignoring that relativistic effects make the trend from lithium to caesium become inapplicable at francium. Some of the few properties of francium that have been predicted taking relativity into account are the electron affinity (47.2 kJ/mol) and the enthalpy of dissociation of the Fr2 molecule (42.1 kJ/mol). The CsFr molecule is polarised as CsFr, showing that the 7s subshell of francium is much more strongly affected by relativistic effects than the 6s subshell of caesium. Additionally, francium superoxide (FrO2) is expected to have significant covalent character, unlike the other alkali metal superoxides, because of bonding contributions from the 6p electrons of francium.
Nuclear
| Z |
Alkali metal |
Stable |
Decays |
unstable: italics odd–odd isotopes coloured pink
| ||
|---|---|---|---|---|---|---|
| 3 | lithium | 2 | — | Li |
Li |
|
| 11 | sodium | 1 | — | Na |
||
| 19 | potassium | 2 | 1 | K |
K |
K |
| 37 | rubidium | 1 | 1 | Rb |
Rb |
|
| 55 | caesium | 1 | — | Cs |
||
| 87 | francium | — | — | No primordial isotopes ( Fr is a radiogenic nuclide) | ||
| Radioactive: K, t1/2 1.25 × 10 years; Rb, t1/2 4.9 × 10 years; Fr, t1/2 22.0 min. | ||||||
All the alkali metals have odd atomic numbers; hence, their isotopes must be either odd–odd (both proton and neutron number are odd) or odd–even (proton number is odd, but neutron number is even). Odd–odd nuclei have even mass numbers, whereas odd–even nuclei have odd mass numbers. Odd–odd primordial nuclides are rare because most odd–odd nuclei are highly unstable with respect to beta decay, because the decay products are even–even, and are therefore more strongly bound, due to nuclear pairing effects.
Due to the great rarity of odd–odd nuclei, almost all the primordial isotopes of the alkali metals are odd–even (the exceptions being the light stable isotope lithium-6 and the long-lived radioisotope potassium-40). For a given odd mass number, there can be only a single beta-stable nuclide, since there is not a difference in binding energy between even–odd and odd–even comparable to that between even–even and odd–odd, leaving other nuclides of the same mass number (isobars) free to beta decay toward the lowest-mass nuclide. An effect of the instability of an odd number of either type of nucleons is that odd-numbered elements, such as the alkali metals, tend to have fewer stable isotopes than even-numbered elements. Of the 26 monoisotopic elements that have only a single stable isotope, all but one have an odd atomic number and all but one also have an even number of neutrons. Beryllium is the single exception to both rules, due to its low atomic number.
All of the alkali metals except lithium and caesium have at least one naturally occurring radioisotope: sodium-22 and sodium-24 are trace radioisotopes produced cosmogenically, potassium-40 and rubidium-87 have very long half-lives and thus occur naturally, and all isotopes of francium are radioactive. Caesium was also thought to be radioactive in the early 20th century, although it has no naturally occurring radioisotopes. (Francium had not been discovered yet at that time.) The natural long-lived radioisotope of potassium, potassium-40, makes up about 0.012% of natural potassium, and thus natural potassium is weakly radioactive. This natural radioactivity became a basis for a mistaken claim of the discovery for element 87 (the next alkali metal after caesium) in 1925. Natural rubidium is similarly slightly radioactive, with 27.83% being the long-lived radioisotope rubidium-87.
Caesium-137, with a half-life of 30.17 years, is one of the two principal medium-lived fission products, along with strontium-90, which are responsible for most of the radioactivity of spent nuclear fuel after several years of cooling, up to several hundred years after use. It constitutes most of the radioactivity still left from the Chernobyl accident. Caesium-137 undergoes high-energy beta decay and eventually becomes stable barium-137. It is a strong emitter of gamma radiation. Caesium-137 has a very low rate of neutron capture and cannot be feasibly disposed of in this way, but must be allowed to decay. Caesium-137 has been used as a tracer in hydrologic studies, analogous to the use of tritium. Small amounts of caesium-134 and caesium-137 were released into the environment during nearly all nuclear weapon tests and some nuclear accidents, most notably the Goiânia accident and the Chernobyl disaster. As of 2005, caesium-137 is the principal source of radiation in the zone of alienation around the Chernobyl nuclear power plant. Its chemical properties as one of the alkali metals make it one of the most problematic of the short-to-medium-lifetime fission products because it easily moves and spreads in nature due to the high water solubility of its salts, and is taken up by the body, which mistakes it for its essential congeners sodium and potassium.
Periodic trends
The alkali metals are more similar to each other than the elements in any other group are to each other. For instance, when moving down the table, all known alkali metals show increasing atomic radius, decreasing electronegativity, increasing reactivity, and decreasing melting and boiling points as well as heats of fusion and vaporisation. In general, their densities increase when moving down the table, with the exception that potassium is less dense than sodium.
Atomic and ionic radii

The atomic radii of the alkali metals increase going down the group. Because of the shielding effect, when an atom has more than one electron shell, each electron feels electric repulsion from the other electrons as well as electric attraction from the nucleus. In the alkali metals, the outermost electron only feels a net charge of +1, as some of the nuclear charge (which is equal to the atomic number) is cancelled by the inner electrons; the number of inner electrons of an alkali metal is always one less than the nuclear charge. Therefore, the only factor which affects the atomic radius of the alkali metals is the number of electron shells. Since this number increases down the group, the atomic radius must also increase down the group.
The ionic radii of the alkali metals are much smaller than their atomic radii. This is because the outermost electron of the alkali metals is in a different electron shell than the inner electrons, and thus when it is removed the resulting atom has one fewer electron shell and is smaller. Additionally, the effective nuclear charge has increased, and thus the electrons are attracted more strongly towards the nucleus and the ionic radius decreases.
First ionisation energy

The first ionisation energy of an element or molecule is the energy required to move the most loosely held electron from one mole of gaseous atoms of the element or molecules to form one mole of gaseous ions with electric charge +1. The factors affecting the first ionisation energy are the nuclear charge, the amount of shielding by the inner electrons and the distance from the most loosely held electron from the nucleus, which is always an outer electron in main group elements. The first two factors change the effective nuclear charge the most loosely held electron feels. Since the outermost electron of alkali metals always feels the same effective nuclear charge (+1), the only factor which affects the first ionisation energy is the distance from the outermost electron to the nucleus. Since this distance increases down the group, the outermost electron feels less attraction from the nucleus and thus the first ionisation energy decreases. This trend is broken in francium due to the relativistic stabilisation and contraction of the 7s orbital, bringing francium's valence electron closer to the nucleus than would be expected from non-relativistic calculations. This makes francium's outermost electron feel more attraction from the nucleus, increasing its first ionisation energy slightly beyond that of caesium.
The second ionisation energy of the alkali metals is much higher than the first as the second-most loosely held electron is part of a fully filled electron shell and is thus difficult to remove.
Reactivity
The reactivities of the alkali metals increase going down the group. This is the result of a combination of two factors: the first ionisation energies and atomisation energies of the alkali metals. Because the first ionisation energy of the alkali metals decreases down the group, it is easier for the outermost electron to be removed from the atom and participate in chemical reactions, thus increasing reactivity down the group. The atomisation energy measures the strength of the metallic bond of an element, which falls down the group as the atoms increase in radius and thus the metallic bond must increase in length, making the delocalised electrons further away from the attraction of the nuclei of the heavier alkali metals. Adding the atomisation and first ionisation energies gives a quantity closely related to (but not equal to) the activation energy of the reaction of an alkali metal with another substance. This quantity decreases going down the group, and so does the activation energy; thus, chemical reactions can occur faster and the reactivity increases down the group.
Electronegativity
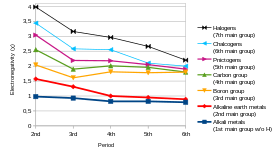
Electronegativity is a chemical property that describes the tendency of an atom or a functional group to attract electrons (or electron density) towards itself. If the bond between sodium and chlorine in sodium chloride were covalent, the pair of shared electrons would be attracted to the chlorine because the effective nuclear charge on the outer electrons is +7 in chlorine but is only +1 in sodium. The electron pair is attracted so close to the chlorine atom that they are practically transferred to the chlorine atom (an ionic bond). However, if the sodium atom was replaced by a lithium atom, the electrons will not be attracted as close to the chlorine atom as before because the lithium atom is smaller, making the electron pair more strongly attracted to the closer effective nuclear charge from lithium. Hence, the larger alkali metal atoms (further down the group) will be less electronegative as the bonding pair is less strongly attracted towards them. As mentioned previously, francium is expected to be an exception.
Because of the higher electronegativity of lithium, some of its compounds have a more covalent character. For example, lithium iodide (LiI) will dissolve in organic solvents, a property of most covalent compounds. Lithium fluoride (LiF) is the only alkali halide that is not soluble in water, and lithium hydroxide (LiOH) is the only alkali metal hydroxide that is not deliquescent.
Melting and boiling points
The melting point of a substance is the point where it changes state from solid to liquid while the boiling point of a substance (in liquid state) is the point where the vapour pressure of the liquid equals the environmental pressure surrounding the liquid and all the liquid changes state to gas. As a metal is heated to its melting point, the metallic bonds keeping the atoms in place weaken so that the atoms can move around, and the metallic bonds eventually break completely at the metal's boiling point. Therefore, the falling melting and boiling points of the alkali metals indicate that the strength of the metallic bonds of the alkali metals decreases down the group. This is because metal atoms are held together by the electromagnetic attraction from the positive ions to the delocalised electrons. As the atoms increase in size going down the group (because their atomic radius increases), the nuclei of the ions move further away from the delocalised electrons and hence the metallic bond becomes weaker so that the metal can more easily melt and boil, thus lowering the melting and boiling points. The increased nuclear charge is not a relevant factor due to the shielding effect.
Density
The alkali metals all have the same crystal structure (body-centred cubic) and thus the only relevant factors are the number of atoms that can fit into a certain volume and the mass of one of the atoms, since density is defined as mass per unit volume. The first factor depends on the volume of the atom and thus the atomic radius, which increases going down the group; thus, the volume of an alkali metal atom increases going down the group. The mass of an alkali metal atom also increases going down the group. Thus, the trend for the densities of the alkali metals depends on their atomic weights and atomic radii; if figures for these two factors are known, the ratios between the densities of the alkali metals can then be calculated. The resultant trend is that the densities of the alkali metals increase down the table, with an exception at potassium. Due to having the lowest atomic weight and the largest atomic radius of all the elements in their periods, the alkali metals are the least dense metals in the periodic table. Lithium, sodium, and potassium are the only three metals in the periodic table that are less dense than water: in fact, lithium is the least dense known solid at room temperature.
Compounds
The alkali metals form complete series of compounds with all usually encountered anions, which well illustrate group trends. These compounds can be described as involving the alkali metals losing electrons to acceptor species and forming monopositive ions. This description is most accurate for alkali halides and becomes less and less accurate as cationic and anionic charge increase, and as the anion becomes larger and more polarisable. For instance, ionic bonding gives way to metallic bonding along the series NaCl, Na2O, Na2S, Na3P, Na3As, Na3Sb, Na3Bi, Na.
| External videos | |
|---|---|

All the alkali metals react vigorously or explosively with cold water, producing an aqueous solution of a strongly basic alkali metal hydroxide and releasing hydrogen gas. This reaction becomes more vigorous going down the group: lithium reacts steadily with effervescence, but sodium and potassium can ignite, and rubidium and caesium sink in water and generate hydrogen gas so rapidly that shock waves form in the water that may shatter glass containers. When an alkali metal is dropped into water, it produces an explosion, of which there are two separate stages. The metal reacts with the water first, breaking the hydrogen bonds in the water and producing hydrogen gas; this takes place faster for the more reactive heavier alkali metals. Second, the heat generated by the first part of the reaction often ignites the hydrogen gas, causing it to burn explosively into the surrounding air. This secondary hydrogen gas explosion produces the visible flame above the bowl of water, lake or other body of water, not the initial reaction of the metal with water (which tends to happen mostly under water). The alkali metal hydroxides are the most basic known hydroxides.
Recent research has suggested that the explosive behavior of alkali metals in water is driven by a Coulomb explosion rather than solely by rapid generation of hydrogen itself. All alkali metals melt as a part of the reaction with water. Water molecules ionise the bare metallic surface of the liquid metal, leaving a positively charged metal surface and negatively charged water ions. The attraction between the charged metal and water ions will rapidly increase the surface area, causing an exponential increase of ionisation. When the repulsive forces within the liquid metal surface exceeds the forces of the surface tension, it vigorously explodes.
The hydroxides themselves are the most basic hydroxides known, reacting with acids to give salts and with alcohols to give oligomeric alkoxides. They easily react with carbon dioxide to form carbonates or bicarbonates, or with hydrogen sulfide to form sulfides or bisulfides, and may be used to separate thiols from petroleum. They react with amphoteric oxides: for example, the oxides of aluminium, zinc, tin, and lead react with the alkali metal hydroxides to give aluminates, zincates, stannates, and plumbates. Silicon dioxide is acidic, and thus the alkali metal hydroxides can also attack silicate glass.
Intermetallic compounds

The alkali metals form many intermetallic compounds with each other and the elements from groups 2 to 13 in the periodic table of varying stoichiometries, such as the sodium amalgams with mercury, including Na5Hg8 and Na3Hg. Some of these have ionic characteristics: taking the alloys with gold, the most electronegative of metals, as an example, NaAu and KAu are metallic, but RbAu and CsAu are semiconductors. NaK is an alloy of sodium and potassium that is very useful because it is liquid at room temperature, although precautions must be taken due to its extreme reactivity towards water and air. The eutectic mixture melts at −12.6 °C. An alloy of 41% caesium, 47% sodium, and 12% potassium has the lowest known melting point of any metal or alloy, −78 °C.
Compounds with the group 13 elements
The intermetallic compounds of the alkali metals with the heavier group 13 elements (aluminium, gallium, indium, and thallium), such as NaTl, are poor conductors or semiconductors, unlike the normal alloys with the preceding elements, implying that the alkali metal involved has lost an electron to the Zintl anions involved. Nevertheless, while the elements in group 14 and beyond tend to form discrete anionic clusters, group 13 elements tend to form polymeric ions with the alkali metal cations located between the giant ionic lattice. For example, NaTl consists of a polymeric anion (—Tl—)n with a covalent diamond cubic structure with Na ions located between the anionic lattice. The larger alkali metals cannot fit similarly into an anionic lattice and tend to force the heavier group 13 elements to form anionic clusters.
Boron is a special case, being the only nonmetal in group 13. The alkali metal borides tend to be boron-rich, involving appreciable boron–boron bonding involving deltahedral structures, and are thermally unstable due to the alkali metals having a very high vapour pressure at elevated temperatures. This makes direct synthesis problematic because the alkali metals do not react with boron below 700 °C, and thus this must be accomplished in sealed containers with the alkali metal in excess. Furthermore, exceptionally in this group, reactivity with boron decreases down the group: lithium reacts completely at 700 °C, but sodium at 900 °C and potassium not until 1200 °C, and the reaction is instantaneous for lithium but takes hours for potassium. Rubidium and caesium borides have not even been characterised. Various phases are known, such as LiB10, NaB6, NaB15, and KB6. Under high pressure the boron–boron bonding in the lithium borides changes from following Wade's rules to forming Zintl anions like the rest of group 13.
Compounds with the group 14 elements
Lithium and sodium react with carbon to form acetylides, Li2C2 and Na2C2, which can also be obtained by reaction of the metal with acetylene. Potassium, rubidium, and caesium react with graphite; their atoms are intercalated between the hexagonal graphite layers, forming graphite intercalation compounds of formulae MC60 (dark grey, almost black), MC48 (dark grey, almost black), MC36 (blue), MC24 (steel blue), and MC8 (bronze) (M = K, Rb, or Cs). These compounds are over 200 times more electrically conductive than pure graphite, suggesting that the valence electron of the alkali metal is transferred to the graphite layers (e.g. MC−8). Upon heating of KC8, the elimination of potassium atoms results in the conversion in sequence to KC24, KC36, KC48 and finally KC60. KC8 is a very strong reducing agent and is pyrophoric and explodes on contact with water. While the larger alkali metals (K, Rb, and Cs) initially form MC8, the smaller ones initially form MC6, and indeed they require reaction of the metals with graphite at high temperatures around 500 °C to form. Apart from this, the alkali metals are such strong reducing agents that they can even reduce buckminsterfullerene to produce solid fullerides MnC60; sodium, potassium, rubidium, and caesium can form fullerides where n = 2, 3, 4, or 6, and rubidium and caesium additionally can achieve n = 1.
When the alkali metals react with the heavier elements in the carbon group (silicon, germanium, tin, and lead), ionic substances with cage-like structures are formed, such as the silicides M4Si4 (M = K, Rb, or Cs), which contains M and tetrahedral Si4−4 ions. The chemistry of alkali metal germanides, involving the germanide ion Ge and other cluster (Zintl) ions such as Ge2−4, Ge4−9, Ge2−9, and [(Ge9)2], is largely analogous to that of the corresponding silicides. Alkali metal stannides are mostly ionic, sometimes with the stannide ion (Sn), and sometimes with more complex Zintl ions such as Sn4−9, which appears in tetrapotassium nonastannide (K4Sn9). The monatomic plumbide ion (Pb) is unknown, and indeed its formation is predicted to be energetically unfavourable; alkali metal plumbides have complex Zintl ions, such as Pb4−9. These alkali metal germanides, stannides, and plumbides may be produced by reducing germanium, tin, and lead with sodium metal in liquid ammonia.
Nitrides and pnictides

Lithium, the lightest of the alkali metals, is the only alkali metal which reacts with nitrogen at standard conditions, and its nitride is the only stable alkali metal nitride. Nitrogen is an unreactive gas because breaking the strong triple bond in the dinitrogen molecule (N2) requires a lot of energy. The formation of an alkali metal nitride would consume the ionisation energy of the alkali metal (forming M ions), the energy required to break the triple bond in N2 and the formation of N ions, and all the energy released from the formation of an alkali metal nitride is from the lattice energy of the alkali metal nitride. The lattice energy is maximised with small, highly charged ions; the alkali metals do not form highly charged ions, only forming ions with a charge of +1, so only lithium, the smallest alkali metal, can release enough lattice energy to make the reaction with nitrogen exothermic, forming lithium nitride. The reactions of the other alkali metals with nitrogen would not release enough lattice energy and would thus be endothermic, so they do not form nitrides at standard conditions. Sodium nitride (Na3N) and potassium nitride (K3N), while existing, are extremely unstable, being prone to decomposing back into their constituent elements, and cannot be produced by reacting the elements with each other at standard conditions. Steric hindrance forbids the existence of rubidium or caesium nitride. However, sodium and potassium form colourless azide salts involving the linear N−3 anion; due to the large size of the alkali metal cations, they are thermally stable enough to be able to melt before decomposing.
All the alkali metals react readily with phosphorus and arsenic to form phosphides and arsenides with the formula M3Pn (where M represents an alkali metal and Pn represents a pnictogen – phosphorus, arsenic, antimony, or bismuth). This is due to the greater size of the P and As ions, so that less lattice energy needs to be released for the salts to form. These are not the only phosphides and arsenides of the alkali metals: for example, potassium has nine different known phosphides, with formulae K3P, K4P3, K5P4, KP, K4P6, K3P7, K3P11, KP10.3, and KP15. While most metals form arsenides, only the alkali and alkaline earth metals form mostly ionic arsenides. The structure of Na3As is complex with unusually short Na–Na distances of 328–330 pm which are shorter than in sodium metal, and this indicates that even with these electropositive metals the bonding cannot be straightforwardly ionic. Other alkali metal arsenides not conforming to the formula M3As are known, such as LiAs, which has a metallic lustre and electrical conductivity indicating the presence of some metallic bonding. The antimonides are unstable and reactive as the Sb ion is a strong reducing agent; reaction of them with acids form the toxic and unstable gas stibine (SbH3). Indeed, they have some metallic properties, and the alkali metal antimonides of stoichiometry MSb involve antimony atoms bonded in a spiral Zintl structure. Bismuthides are not even wholly ionic; they are intermetallic compounds containing partially metallic and partially ionic bonds.


![{\displaystyle {\ce {LiR\ +\ Ni(CO)4\ \longrightarrow Li^{+}[RCONi(CO)3]^{-}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b1783ddc7161bab3c63eb4c179496f65fc6ece16)
![{\displaystyle {\ce {Li^{+}[RCONi(CO)3]^{-}->[{\ce {H^{+}}}][{\ce {solvent}}]\ Li^{+}\ +\ RCHO\ +\ [(solvent)Ni(CO)3]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/048a72890ac48383f7155a9f800660ab7b919d38)
![{\displaystyle {\ce {Li^{+}[RCONi(CO)3]^{-}->[{\ce {R^{'}Br}}][{\ce {solvent}}]\ Li^{+}\ +\ RR^{'}CO\ +\ [(solvent)Ni(CO)3]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7ccae3545edde30fdaec9ebc9a5dde6cbd28d06d)
![{\displaystyle {\ce {2 Na \ + H2 \ ->[{\ce {\Delta}}] \ 2 NaH}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/31df93df654a7b734a6adf394ab9bc5b69484a3a)


![{\displaystyle {\ce {2Na\ +\ 2C2H2\ ->[{\ce {150\ ^{o}C}}]\ 2NaC2H\ +\ H2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a763ecb3a7dd778b986b68bdebab4f5b5a28ce7a)
![{\displaystyle {\ce {2Na\ +\ 2NaC2H\ ->[{\ce {220\ ^{o}C}}]\ 2Na2C2\ +\ H2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5dee10d9e2a8694d6b12badfe5ee74aa5e4d66c0)