Extinction Of The Non-bird Dinosaurs
As originally proposed in 1980 by a team of scientists led by Luis Alvarez and his son Walter, it is now generally thought that the K–Pg extinction was caused by the impact of a massive asteroid 10 to 15 km (6 to 9 mi) wide, 66 million years ago, which devastated the global environment, mainly through a lingering impact winter which halted photosynthesis in plants and plankton. The impact hypothesis, also known as the Alvarez hypothesis, was bolstered by the discovery of the 180 km (112 mi) Chicxulub crater in the Gulf of Mexico's Yucatán Peninsula in the early 1990s, which provided conclusive evidence that the K–Pg boundary clay represented debris from an asteroid impact. The fact that the extinctions occurred simultaneously provides strong evidence that they were caused by the asteroid. A 2016 drilling project into the Chicxulub peak ring confirmed that the peak ring comprised granite ejected within minutes from deep in the earth, but contained hardly any gypsum, the usual sulfate-containing sea floor rock in the region: the gypsum would have vaporized and dispersed as an aerosol into the atmosphere, causing longer-term effects on the climate and food chain. In October 2019, researchers asserted that the event rapidly acidified the oceans and produced long-lasting effects on the climate, detailing the mechanisms of the mass extinction.
Other causal or contributing factors to the extinction may have been the Deccan Traps and other volcanic eruptions, climate change, and sea level change. However, in January 2020, scientists reported that climate-modeling of the extinction event favored the asteroid impact and not volcanism.
A wide range of terrestrial species perished in the K–Pg extinction, the best-known being the non-avian dinosaurs, along with many mammals, birds, lizards, insects, plants, and all the pterosaurs. In the oceans, the K–Pg extinction killed off plesiosaurs and mosasaurs and devastated teleost fish, sharks, mollusks (especially ammonites, which became extinct), and many species of plankton. It is estimated that 75% or more of all species on Earth vanished. However, the extinction also provided evolutionary opportunities: in its wake, many groups underwent remarkable adaptive radiation—sudden and prolific divergence into new forms and species within the disrupted and emptied ecological niches. Mammals in particular diversified in the Paleogene, evolving new forms such as horses, whales, bats, and primates. The surviving group of dinosaurs were avians, a few species of ground and water fowl, which radiated into all modern species of birds. Among other groups, teleost fish and perhaps lizards also radiated.
Extinction patterns
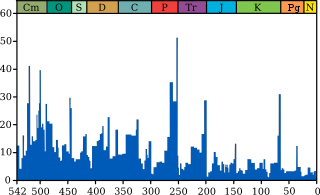
The K–Pg extinction event was severe, global, rapid, and selective, eliminating a vast number of species. Based on marine fossils, it is estimated that 75% or more of all species became extinct.
The event appears to have affected all continents at the same time. Non-avian dinosaurs, for example, are known from the Maastrichtian of North America, Europe, Asia, Africa, South America, and Antarctica, but are unknown from the Cenozoic anywhere in the world. Similarly, fossil pollen shows devastation of the plant communities in areas as far apart as New Mexico, Alaska, China, and New Zealand. Nevertheless, high latitudes appear to have been less strongly affected than low latitudes.
Despite the event's severity, there was significant variability in the rate of extinction between and within different clades. Species that depended on photosynthesis declined or became extinct as atmospheric particles blocked sunlight and reduced the solar energy reaching the ground. This plant extinction caused a major reshuffling of the dominant plant groups. Omnivores, insectivores, and carrion-eaters survived the extinction event, perhaps because of the increased availability of their food sources. Neither strictly herbivorous nor strictly carnivorous mammals seem to have survived. Rather, the surviving mammals and birds fed on insects, worms, and snails, which in turn fed on detritus (dead plant and animal matter).
In stream communities and lake ecosystems, few animal groups became extinct, including large forms like crocodyliforms and champsosaurs, because such communities rely less directly on food from living plants, and more on detritus washed in from the land, protecting them from extinction. Modern crocodilians can live as scavengers and survive for months without food, and their young are small, grow slowly, and feed largely on invertebrates and dead organisms for their first few years. These characteristics have been linked to crocodilian survival at the end of the Cretaceous. Similar, but more complex patterns have been found in the oceans. Extinction was more severe among animals living in the water column than among animals living on or in the sea floor. Animals in the water column are almost entirely dependent on primary production from living phytoplankton, while animals on the ocean floor always or sometimes feed on detritus. Coccolithophorids and mollusks (including ammonites, rudists, freshwater snails, and mussels), and those organisms whose food chain included these shell builders, became extinct or suffered heavy losses. For example, it is thought that ammonites were the principal food of mosasaurs, a group of giant marine reptiles that became extinct at the boundary.
The K–Pg extinction had a profound effect on the evolution of life on Earth. The elimination of dominant Cretaceous groups allowed other organisms to take their place, causing a remarkable amount of species diversification during the Paleogene Period. After the K–Pg extinction event, biodiversity required substantial time to recover, despite the existence of abundant vacant ecological niches. Evidence from the Salamanca Formation suggests that biotic recovery was more rapid in the Southern Hemisphere than in the Northern Hemisphere.
Microbiota
The K–Pg boundary represents one of the most dramatic turnovers in the fossil record for various calcareous nanoplankton that formed the calcium deposits for which the Cretaceous is named. The turnover in this group is clearly marked at the species level. Statistical analysis of marine losses at this time suggests that the decrease in diversity was caused more by a sharp increase in extinctions than by a decrease in speciation. Major spatial differences existed in calcareous nannoplankton diversity patterns; in the Southern Hemisphere, the extinction was less severe and recovery occurred much faster than in the Northern Hemisphere. Following the extinction, survivor communities dominated for several hundred thousand years. The North Pacific acted as a diversity hotspot from which later nannoplankton communities radiated as they replaced survivor faunas across the globe.
The K–Pg boundary record of dinoflagellates is not so well understood, mainly because only microbial cysts provide a fossil record, and not all dinoflagellate species have cyst-forming stages, which likely causes diversity to be underestimated. Recent studies indicate that there were no major shifts in dinoflagellates through the boundary layer. There were blooms of the taxa Thoracosphaera operculata and Braarudosphaera bigelowii at the boundary.
Radiolaria have left a geological record since at least the Ordovician times, and their mineral fossil skeletons can be tracked across the K–Pg boundary. There is no evidence of mass extinction of these organisms, and there is support for high productivity of these species in southern high latitudes as a result of cooling temperatures in the early Paleocene. Approximately 46% of diatom species survived the transition from the Cretaceous to the Upper Paleocene, a significant turnover in species but not a catastrophic extinction.
The occurrence of planktonic foraminifera across the K–Pg boundary has been studied since the 1930s. Research spurred by the possibility of an impact event at the K–Pg boundary resulted in numerous publications detailing planktonic foraminiferal extinction at the boundary; there is ongoing debate between groups which think the evidence indicates substantial extinction of these species at the K–Pg boundary, and those who think the evidence supports a gradual extinction through the boundary. There is strong evidence that local conditions heavily influenced diversity changes in planktonic foraminifera. Low and mid-latitude communities of planktonic foraminifera experienced high extinction rates, while high latitude faunas were relatively unaffected.
Numerous species of benthic foraminifera became extinct during the event, presumably because they depend on organic debris for nutrients, while biomass in the ocean is thought to have decreased. As the marine microbiota recovered, it is thought that increased speciation of benthic foraminifera resulted from the increase in food sources. In some areas, such as Texas, benthic foraminifera show no sign of any major extinction event, however. Phytoplankton recovery in the early Paleocene provided the food source to support large benthic foraminiferal assemblages, which are mainly detritus-feeding. Ultimate recovery of the benthic populations occurred over several stages lasting several hundred thousand years into the early Paleocene.
Marine invertebrates

There is significant variation in the fossil record as to the extinction rate of marine invertebrates across the K–Pg boundary. The apparent rate is influenced by a lack of fossil records, rather than extinctions.
Ostracods, a class of small crustaceans that were prevalent in the upper Maastrichtian, left fossil deposits in a variety of locations. A review of these fossils shows that ostracod diversity was lower in the Paleocene than any other time in the Cenozoic. Current research cannot ascertain whether the extinctions occurred prior to, or during, the boundary interval. Ostracods that were heavily sexually selected were more vulnerable to extinction, and ostracod sexual dimorphism was significantly rarer following the mass extinction.
Among decapods, extinction patterns were highly heterogeneous and cannot be neatly attributed to any particular factor. Decapods that inhabited the Western Interior Seaway were especially hard-hit, while other regions of the world's oceans were refugia that increased chances of survival into the Palaeocene. Among retroplumid crabs, the genus Costacopluma was a notable survivor.
Approximately 60% of late-Cretaceous scleractinian coral genera failed to cross the K–Pg boundary into the Paleocene. Further analysis of the coral extinctions shows that approximately 98% of colonial species, ones that inhabit warm, shallow tropical waters, became extinct. The solitary corals, which generally do not form reefs and inhabit colder and deeper (below the photic zone) areas of the ocean were less impacted by the K–Pg boundary. Colonial coral species rely upon symbiosis with photosynthetic algae, which collapsed due to the events surrounding the K–Pg boundary, but the use of data from coral fossils to support K–Pg extinction and subsequent Paleocene recovery, must be weighed against the changes that occurred in coral ecosystems through the K–Pg boundary.
Most species of brachiopods, a small phylum of marine invertebrates, survived the K–Pg extinction event and diversified during the early Paleocene.
The numbers bivalve genera exhibited significant diminution after the K–Pg boundary. Entire groups of bivalves, including rudists (reef-building clams) and inoceramids (giant relatives of modern scallops), became extinct at the K–Pg boundary, with the gradual extinction of most inoceramid bivalves beginning well before the K–Pg boundary. Deposit feeders were the most common bivalves in the catastrophe's aftermath. Abundance was not a factor that affected whether a bivalve taxon went extinct, according to evidence from North America. Veneroid bivalves developed deeper burrowing habitats as the recovery from the crisis ensued.

Except for nautiloids (represented by the modern order Nautilida) and coleoids (which had already diverged into modern octopodes, squids, and cuttlefish) all other species of the molluscan class Cephalopoda became extinct at the K–Pg boundary. These included the ecologically significant belemnoids, as well as the ammonoids, a group of highly diverse, numerous, and widely distributed shelled cephalopods. The extinction of belemnites enabled surviving cephalopod clades to fill their niches. Ammonite genera became extinct at or near the K–Pg boundary; there was a smaller and slower extinction of ammonite genera prior to the boundary associated with a late Cretaceous marine regression, and a small, gradual reduction in ammonite diversity occurred throughout the very late Cretaceous. Researchers have pointed out that the reproductive strategy of the surviving nautiloids, which rely upon few and larger eggs, played a role in outsurviving their ammonoid counterparts through the extinction event. The ammonoids utilized a planktonic strategy of reproduction (numerous eggs and planktonic larvae), which would have been devastated by the K–Pg extinction event. Additional research has shown that subsequent to this elimination of ammonoids from the global biota, nautiloids began an evolutionary radiation into shell shapes and complexities theretofore known only from ammonoids.
Approximately 35% of echinoderm genera became extinct at the K–Pg boundary, although taxa that thrived in low-latitude, shallow-water environments during the late Cretaceous had the highest extinction rate. Mid-latitude, deep-water echinoderms were much less affected at the K–Pg boundary. The pattern of extinction points to habitat loss, specifically the drowning of carbonate platforms, the shallow-water reefs in existence at that time, by the extinction event. Atelostomatans were affected by the Lilliput effect.
Terrestrial invertebrates
Insect damage to the fossilized leaves of flowering plants from fourteen sites in North America was used as a proxy for insect diversity across the K–Pg boundary and analyzed to determine the rate of extinction. Researchers found that Cretaceous sites, prior to the extinction event, had rich plant and insect-feeding diversity. During the early Paleocene, flora were relatively diverse with little predation from insects, even 1.7 million years after the extinction event. Studies of the size of the ichnotaxon Naktodemasis bowni, produced by either cicada nymphs or beetle larvae, over the course of the K-Pg transition show that the Lilliput effect occurred in terrestrial invertebrates thanks to the extinction event.
The extinction event produced major changes in Paleogene insect communities. Many groups of ants were present in the Cretaceous, but in the Eocene ants became dominant and diverse, with larger colonies. Butterflies diversified as well, perhaps to take the place of leaf-eating insects wiped out by the extinction. The advanced mound-building termites, Termitidae, also appear to have risen in importance.
Fish
There are fossil records of jawed fishes across the K–Pg boundary, which provide good evidence of extinction patterns of these classes of marine vertebrates. While the deep-sea realm was able to remain seemingly unaffected, there was an equal loss between the open marine apex predators and the durophagous demersal feeders on the continental shelf. Within cartilaginous fish, approximately 7 out of the 41 families of neoselachians (modern sharks, skates, and rays) disappeared after this event and batoids (skates and rays) lost nearly all the identifiable species, while more than 90% of teleost fish (bony fish) families survived.
In the Maastrichtian age, 28 shark families and 13 batoid families thrived, of which 25 and 9, respectively, survived the K–T boundary event. Forty-seven of all neoselachian genera cross the K–T boundary, with 85% being sharks. Batoids display with 15%, a comparably low survival rate. Among elasmobranchs, those species that inhabited higher latitudes and lived pelagic lifestyles were more likely to survive, whereas epibenthic lifestyles and durophagy were strongly associated with the likelihood of perishing during the extinction event.
There is evidence of a mass extinction of bony fishes at a fossil site immediately above the K–Pg boundary layer on Seymour Island near Antarctica, apparently precipitated by the K–Pg extinction event; the marine and freshwater environments of fishes mitigated the environmental effects of the extinction event. The result was Patterson's Gap, a period in the earliest part of the Cenozoic of decreased acanthomorph diversity, although acanthomorphs diversified rapidly after the extinction. Teleost fish diversified explosively after the mass extinction, filling the niches left vacant by the extinction. Groups appearing in the Paleocene and Eocene epochs include billfish, tunas, eels, and flatfish.
Amphibians
There is limited evidence for extinction of amphibians at the K–Pg boundary. A study of fossil vertebrates across the K–Pg boundary in Montana concluded that no species of amphibian became extinct. Yet there are several species of Maastrichtian amphibian, not included as part of this study, which are unknown from the Paleocene. These include the frog Theatonius lancensis and the albanerpetontid Albanerpeton galaktion; therefore, some amphibians do seem to have become extinct at the boundary. The relatively low levels of extinction seen among amphibians probably reflect the low extinction rates seen in freshwater animals. Following the mass extinction, frogs radiated substantially, with 88% of modern anuran diversity being traced back to three lineages of frogs that evolved after the cataclysm.
Reptiles
Choristoderes
The choristoderes (a group of semi-aquatic diapsids of uncertain position) survived across the K–Pg boundary subsequently becoming extinct in the Miocene. The gharial-like choristodere genus Champsosaurus' palatal teeth suggest that there were dietary changes among the various species across the K–Pg event.
Turtles
More than 80% of Cretaceous turtle species passed through the K–Pg boundary. All six turtle families in existence at the end of the Cretaceous survived into the Paleogene and are represented by living species. Analysis of turtle survivorship in the Hell Creek Formation shows a minimum of 75% of turtle species survived. Following the extinction event, turtle diversity exceeded pre-extinction levels in the Danian of North America, although in South America it remained diminished. European turtles likewise recovered rapidly following the mass extinction.
Lepidosauria
The rhynchocephalians which were a globally distributed and diverse group of lepidosaurians during the early Mesozoic, had begun to decline by the mid-Cretaceous, although they remained successful in the Late Cretaceous of southern South America. They are represented today by a single species, the tuatara (Sphenodon punctatus) found in New Zealand. Outside of New Zealand, one rhynchocephalian is known to have crossed the K-Pg boundary, Kawasphenodon peligrensis, known from the earliest Paleocene (Danian) of Patagonia.
The order Squamata comprising lizards and snakes first diversified during the Jurassic and continued to diversify throughout the Cretaceous. They are currently the most successful and diverse group of living reptiles, with more than 10,000 extant species. The only major group of terrestrial lizards to go extinct at the end of the Cretaceous were the polyglyphanodontians, a diverse group of mainly herbivorous lizards known predominantly from the Northern Hemisphere. The mosasaurs, a diverse group of large predatory marine reptiles, also became extinct. Fossil evidence indicates that squamates generally suffered very heavy losses in the K–Pg event, only recovering 10 million years after it. The extinction of Cretaceous lizards and snakes may have led to the evolution of modern groups such as iguanas, monitor lizards, and boas. The diversification of crown group snakes has been linked to the biotic recovery in the aftermath of the K-Pg extinction event. Pan-Gekkotans weathered the extinction event well, with multiple lineages likely surviving.
Marine reptiles
∆Ca values indicate that prior to the mass extinction, marine reptiles at the top of food webs were feeding on only one source of calcium, suggesting their populations exhibited heightened vulnerability to extinctions at the terminus of the Cretaceous. Along with the aforementioned mosasaurs, plesiosaurs, represented by the families Elasmosauridae and Polycotylidae, became extinct during the event. The ichthyosaurs had disappeared from fossil record tens of millions of years prior to the K-Pg extinction event.
Crocodyliforms
Ten families of crocodilians or their close relatives are represented in the Maastrichtian fossil records, of which five died out prior to the K–Pg boundary. Five families have both Maastrichtian and Paleocene fossil representatives. All of the surviving families of crocodyliforms inhabited freshwater and terrestrial environments—except for the Dyrosauridae, which lived in freshwater and marine locations. Approximately 50% of crocodyliform representatives survived across the K–Pg boundary, the only apparent trend being that no large crocodiles survived. Crocodyliform survivability across the boundary may have resulted from their aquatic niche and ability to burrow, which reduced susceptibility to negative environmental effects at the boundary. Jouve and colleagues suggested in 2008 that juvenile marine crocodyliforms lived in freshwater environments as do modern marine crocodile juveniles, which would have helped them survive where other marine reptiles became extinct; freshwater environments were not so strongly affected by the K–Pg extinction event as marine environments were. Among the terrestrial clade Notosuchia, only the family Sebecidae survived; the exact reasons for this pattern are not known. Sebecids were large terrestrial predators, are known from the Eocene of Europe, and would survive in South America into the Miocene. Tethysuchians radiated explosively after the extinction event.
Pterosaurs
Two families of pterosaurs, Azhdarchidae and Nyctosauridae, were definitely present in the Maastrichtian, and they likely became extinct at the K–Pg boundary. Several other pterosaur lineages may have been present during the Maastrichtian, such as the ornithocheirids, pteranodontids, a possible tapejarid, a possible thalassodromid and a basal toothed taxon of uncertain affinities, though they are represented by fragmentary remains that are difficult to assign to any given group. While this was occurring, modern birds were undergoing diversification; traditionally it was thought that they replaced archaic birds and pterosaur groups, possibly due to direct competition, or they simply filled empty niches, but there is no correlation between pterosaur and avian diversities that are conclusive to a competition hypothesis, and small pterosaurs were present in the Late Cretaceous. At least some niches previously held by birds were reclaimed by pterosaurs prior to the K–Pg event.
Non-avian dinosaurs

Scientists agree that all non-avian dinosaurs became extinct at the K–Pg boundary. The dinosaur fossil record has been interpreted to show both a decline in diversity and no decline in diversity during the last few million years of the Cretaceous, and it may be that the quality of the dinosaur fossil record is simply not good enough to permit researchers to distinguish between the options. There is no evidence that late Maastrichtian non-avian dinosaurs could burrow, swim, or dive, which suggests they were unable to shelter themselves from the worst parts of any environmental stress that occurred at the K–Pg boundary. It is possible that small dinosaurs (other than birds) did survive, but they would have been deprived of food, as herbivorous dinosaurs would have found plant material scarce and carnivores would have quickly found prey in short supply.
The growing consensus about the endothermy of dinosaurs (see dinosaur physiology) helps to understand their full extinction in contrast with their close relatives, the crocodilians. Ectothermic ("cold-blooded") crocodiles have very limited needs for food (they can survive several months without eating), while endothermic ("warm-blooded") animals of similar size need much more food to sustain their faster metabolism. Thus, under the circumstances of food chain disruption previously mentioned, non-avian dinosaurs died out, while some crocodiles survived. In this context, the survival of other endothermic animals, such as some birds and mammals, could be due, among other reasons, to their smaller needs for food, related to their small size at the extinction epoch. Prolonged cold is unlikely to have been a reason for the extinction of non-avian dinosaurs given the adaptations of many dinosaurs to cold environments.
Whether the extinction occurred gradually or suddenly has been debated, as both views have support from the fossil record. A highly informative sequence of dinosaur-bearing rocks from the K–Pg boundary is found in western North America, particularly the late Maastrichtian-age Hell Creek Formation of Montana. Comparison with the older Judith River Formation (Montana) and Dinosaur Park Formation (Alberta), which both date from approximately 75 Ma, provides information on the changes in dinosaur populations over the last 10 million years of the Cretaceous. These fossil beds are geographically limited, covering only part of one continent. The middle–late Campanian formations show a greater diversity of dinosaurs than any other single group of rocks. The late Maastrichtian rocks contain the largest members of several major clades: Tyrannosaurus, Ankylosaurus, Pachycephalosaurus, Triceratops, and Torosaurus, which suggests food was plentiful immediately prior to the extinction. A study of 29 fossil sites in Catalan Pyrenees of Europe in 2010 supports the view that dinosaurs there had great diversity until the asteroid impact, with more than 100 living species. More recent research indicates that this figure is obscured by taphonomic biases and the sparsity of the continental fossil record. The results of this study, which were based on estimated real global biodiversity, showed that between 628 and 1,078 non-avian dinosaur species were alive at the end of the Cretaceous and underwent sudden extinction after the Cretaceous–Paleogene extinction event. Alternatively, interpretation based on the fossil-bearing rocks along the Red Deer River in Alberta, Canada, supports the gradual extinction of non-avian dinosaurs; during the last 10 million years of the Cretaceous layers there, the number of dinosaur species seems to have decreased from about 45 to approximately 12. Other scientists have made the same assessment following their research.
Several researchers support the existence of Paleocene non-avian dinosaurs. Evidence of this existence is based on the discovery of dinosaur remains in the Hell Creek Formation up to 1.3 m (4.3 ft) above and 40,000 years later than the K–Pg boundary. Pollen samples recovered near a fossilized hadrosaur femur recovered in the Ojo Alamo Sandstone at the San Juan River in Colorado, indicate that the animal lived during the Cenozoic, approximately 64.5 Ma (about 1 million years after the K–Pg extinction event). If their existence past the K–Pg boundary can be confirmed, these hadrosaurids would be considered a dead clade walking. The scientific consensus is that these fossils were eroded from their original locations and then re-buried in much later sediments (also known as reworked fossils).
Birds
Most paleontologists regard birds as the only surviving dinosaurs (see Origin of birds). It is thought that all non-avian theropods became extinct, including then-flourishing groups such as enantiornithines and hesperornithiforms. Several analyses of bird fossils show divergence of species prior to the K–Pg boundary, and that duck, chicken, and ratite bird relatives coexisted with non-avian dinosaurs. Large collections of bird fossils representing a range of different species provide definitive evidence for the persistence of archaic birds to within 300,000 years of the K–Pg boundary. The absence of these birds in the Paleogene is evidence that a mass extinction of archaic birds took place there.
The most successful and dominant group of avialans, enantiornithes, were wiped out. Only a small fraction of ground and water-dwelling Cretaceous bird species survived the impact, giving rise to today's birds. The only bird group known for certain to have survived the K–Pg boundary is the Aves. Avians may have been able to survive the extinction as a result of their abilities to dive, swim, or seek shelter in water and marshlands. Many species of avians can build burrows, or nest in tree holes, or termite nests, all of which provided shelter from the environmental effects at the K–Pg boundary. Long-term survival past the boundary was assured as a result of filling ecological niches left empty by extinction of non-avian dinosaurs. Based on molecular sequencing and fossil dating, many species of birds (the Neoaves group in particular) appeared to radiate after the K–Pg boundary. The open niche space and relative scarcity of predators following the K-Pg extinction allowed for adaptive radiation of various avian groups. Ratites, for example, rapidly diversified in the early Paleogene and are believed to have convergently developed flightlessness at least three to six times, often fulfilling the niche space for large herbivores once occupied by non-avian dinosaurs.
Mammals
Mammalian species began diversifying approximately 30 million years prior to the K–Pg boundary. Diversification of mammals stalled across the boundary. All major Late Cretaceous mammalian lineages, including monotremes (egg-laying mammals), multituberculates, metatherians (which includes modern marsupials), eutherians (which includes modern placentals), meridiolestidans, and gondwanatheres survived the K–Pg extinction event, although they suffered losses. In particular, metatherians largely disappeared from North America, and the Asian deltatheroidans became extinct (aside from the lineage leading to Gurbanodelta). In the Hell Creek beds of North America, at least half of the ten known multituberculate species and all eleven metatherians species are not found above the boundary. Multituberculates in Europe and North America survived relatively unscathed and quickly bounced back in the Paleocene, but Asian forms were devastated, never again to represent a significant component of mammalian fauna. A recent study indicates that metatherians suffered the heaviest losses at the K–Pg event, followed by multituberculates, while eutherians recovered the quickest. K–Pg boundary mammalian species were generally small, comparable in size to rats; this small size would have helped them find shelter in protected environments. It is postulated that some early monotremes, marsupials, and placentals were semiaquatic or burrowing, as there are multiple mammalian lineages with such habits today. Any burrowing or semiaquatic mammal would have had additional protection from K–Pg boundary environmental stresses.
After the K–Pg extinction, mammals evolved to fill the niches left vacant by the dinosaurs. Some research indicates that mammals did not explosively diversify across the K–Pg boundary, despite the ecological niches made available by the extinction of dinosaurs. Several mammalian orders have been interpreted as diversifying immediately after the K–Pg boundary, including Chiroptera (bats) and Cetartiodactyla (a diverse group that today includes whales and dolphins and even-toed ungulates), although recent research concludes that only marsupial orders diversified soon after the K–Pg boundary. However, morphological diversification rates among eutherians after the extinction event were thrice those of before it. Also significant, within the mammalian genera, new species were approximately 9.1% larger after the K–Pg boundary. After about 700,000 years, some mammals had reached 50 kilos (110 pounds), a 100-fold increase over the weight of those which survived the extinction. It is thought that body sizes of placental mammalian survivors evolutionarily increased first, allowing them to fill niches after the extinctions, with brain sizes increasing later in the Eocene.
Terrestrial plants
Plant fossils illustrate the reduction in plant species across the K–Pg boundary. There is overwhelming evidence of global disruption of plant communities at the K–Pg boundary. Extinctions are seen both in studies of fossil pollen, and fossil leaves. In North America, the data suggests massive devastation and mass extinction of plants at the K–Pg boundary sections, although there were substantial megafloral changes before the boundary. In North America, approximately 57% of plant species became extinct. In high southern hemisphere latitudes, such as New Zealand and Antarctica, the mass die-off of flora caused no significant turnover in species, but dramatic and short-term changes in the relative abundance of plant groups. European flora was also less affected, most likely due to its distance from the site of the Chicxulub impact. In northern Alaska and the Anadyr-Koryak region of Russia, the flora was minimally impacted. Another line of evidence of a major floral extinction is that the divergence rate of subviral pathogens of angiosperms sharply decreased, which indicates an enormous reduction in the number of flowering plants. However, phylogenetic evidence shows no mass angiosperm extinction.
Due to the wholesale destruction of plants at the K–Pg boundary, there was a proliferation of saprotrophic organisms, such as fungi, that do not require photosynthesis and use nutrients from decaying vegetation. The dominance of fungal species lasted only a few years while the atmosphere cleared and plenty of organic matter to feed on was present. Once the atmosphere cleared photosynthetic organisms returned – initially ferns and other ground-level plants.
In some regions, the Paleocene recovery of plants began with recolonizations by fern species, represented as a fern spike in the geologic record; this same pattern of fern recolonization was observed after the 1980 Mount St. Helens eruption. Just two species of fern appear to have dominated the landscape for centuries after the event. In the sediments below the K–Pg boundary the dominant plant remains are angiosperm pollen grains, but the boundary layer contains little pollen and is dominated by fern spores. More usual pollen levels gradually resume above the boundary layer. This is reminiscent of areas blighted by modern volcanic eruptions, where the recovery is led by ferns, which are later replaced by larger angiosperm plants. In North American terrestrial sequences, the extinction event is best represented by the marked discrepancy between the rich and relatively abundant late-Maastrichtian pollen record and the post-boundary fern spike.
Polyploidy appears to have enhanced the ability of flowering plants to survive the extinction, probably because the additional copies of the genome such plants possessed allowed them to more readily adapt to the rapidly changing environmental conditions that followed the impact.
Beyond extinction impacts, the event also caused more general changes of flora such as giving rise to neotropical rainforest biomes like the Amazonia, replacing species composition and structure of local forests during ~6 million years of recovery to former levels of plant diversity.
Fungi
While it appears that many fungi were wiped out at the K-Pg boundary, there is some evidence that some fungal species thrived in the years after the extinction event. Microfossils from that period indicate a great increase in fungal spores, long before the resumption of plentiful fern spores in the recovery after the impact. Monoporisporites and hypha are almost exclusive microfossils for a short span during and after the iridium boundary. These saprophytes would not need sunlight, allowing them to survive during a period when the atmosphere was likely clogged with dust and sulfur aerosols.
The proliferation of fungi has occurred after several extinction events, including the Permian–Triassic extinction event, the largest known mass extinction in Earth's history, with up to 96% of all species suffering extinction.
Dating

A 1991 study of fossil leaves dated the extinction-associated freezing to early June. A later study shifted the dating to spring season, based on the osteological evidence and stable isotope records of well-preserved bones of acipenseriform fishes. The study noted that "the palaeobotanical identities, taphonomic inferences and stratigraphic assumptions" for the June dating have since all been refuted. Another modern study opted for the spring–summer range. A study of fossilized fish bones found at Tanis in North Dakota suggests that the Cretaceous-Paleogene mass extinction happened during the Northern Hemisphere spring.
Duration
The extinction's rapidity is a controversial issue because some researchers think the extinction was the result of a sudden event, while others argue that it took place over a long period. The exact length of time is difficult to determine because of the Signor–Lipps effect, where the fossil record is so incomplete that most extinct species probably died out long after the most recent fossil that has been found. Scientists have also found very few continuous beds of fossil-bearing rock that cover a time range from several million years before the K–Pg extinction to several million years after it.
The sedimentation rate and thickness of K–Pg clay from three sites suggest rapid extinction, perhaps over a period of less than 10,000 years. At one site in the Denver Basin of Colorado, after the K–Pg boundary layer was deposited, the fern spike lasted approximately 1,000 years, and no more than 71,000 years; at the same location, the earliest appearance of Cenozoic mammals occurred after approximately 185,000 years, and no more than 570,000 years, "indicating rapid rates of biotic extinction and initial recovery in the Denver Basin during this event." Analysis of the carbon cycle disruptions caused by the impact constrains them to an interval of just 5,000 years. Models presented at the annual meeting of the American Geophysical Union demonstrated that the period of global darkness following the Chicxulub impact would have persisted in the Hell Creek Formation nearly 2 years.
Causes
Chicxulub impact
Evidence for impact


In 1980, a team of researchers consisting of Nobel Prize-winning physicist Luis Alvarez, his son, geologist Walter Alvarez, and chemists Frank Asaro and Helen Michel discovered that sedimentary layers found all over the world at the Cretaceous–Paleogene boundary contain a concentration of iridium many times greater than normal (30, 160, and 20 times in three sections originally studied). Iridium is extremely rare in Earth's crust because it is a siderophile element which mostly sank along with iron into Earth's core during planetary differentiation. Instead, iridium is more common in comets and asteroids. Because of this, the Alvarez team suggested that an asteroid struck the Earth at the time of the K–Pg boundary. There were earlier speculations on the possibility of an impact event, but this was the first hard evidence, and since then, studies have continued to demonstrate elevated iridium levels in association with the K-Pg boundary. This hypothesis was viewed as radical when first proposed, but additional evidence soon emerged. The boundary clay was found to be full of minute spherules of rock, crystallized from droplets of molten rock formed by the impact. Shocked quartz and other minerals were also identified in the K–Pg boundary. The identification of giant tsunami beds along the Gulf Coast and the Caribbean provided more evidence, and suggested that the impact might have occurred nearby, as did the discovery that the K–Pg boundary became thicker in the southern United States, with meter-thick beds of debris occurring in northern New Mexico. A K-Pg boundary "cocktail" of microfossils, lithic fragments, and impact-derived material deposited by gigantic sediment gravity flows was discovered in the Caribbean that served to demarcate the impact. Further research identified the giant Chicxulub crater, buried under Chicxulub on the coast of Yucatán, as the source of the K–Pg boundary clay. Identified in 1990 based on work by geophysicist Glen Penfield in 1978, the crater is oval, with an average diameter of roughly 180 km (110 mi), about the size calculated by the Alvarez team. In March 2010, an international panel of 41 scientists reviewed 20 years of scientific literature and endorsed the asteroid hypothesis, specifically the Chicxulub impact, as the cause of the extinction, ruling out other theories such as massive volcanism. They had determined that a 10-to-15-kilometer-wide (6 to 9 mi) asteroid hurtled into Earth at Chicxulub on Mexico's Yucatán Peninsula. Additional evidence for the impact event is found at the Tanis site in southwestern North Dakota, United States. Tanis is part of the heavily studied Hell Creek Formation, a group of rocks spanning four states in North America renowned for many significant fossil discoveries from the Upper Cretaceous and lower Paleocene. Tanis is an extraordinary and unique site because it appears to record the events from the first minutes until a few hours after the impact of the giant Chicxulub asteroid in extreme detail. Amber from the site has been reported to contain microtektites matching those of the Chicxulub impact event. Some researchers question the interpretation of the findings at the site or are skeptical of the team leader, Robert DePalma, who had not yet received his Ph.D. in geology at the time of the discovery and whose commercial activities have been regarded with suspicion. Furthermore, indirect evidence of an asteroid impact as the cause of the mass extinction comes from patterns of turnover in marine plankton.

Some critics of the impact theory have put forward that the impact precedes the mass extinction by about 300,000 years and thus was not its cause. However, in a 2013 paper, Paul Renne of the Berkeley Geochronology Center dated the impact at 66.043±0.011 million years ago, based on argon–argon dating. He further posits that the mass extinction occurred within 32,000 years of this date. The dating of hydrothermally altered structures around the crater is consistent with this timeline.
In 2007, it was proposed that the impactor belonged to the Baptistina family of asteroids. This link has been doubted, though not disproved, in part because of a lack of observations of the asteroid and its family. It was reported in 2009 that 298 Baptistina does not share the chemical signature of the K–Pg impactor. Further, a 2011 Wide-field Infrared Survey Explorer (WISE) study of reflected light from the asteroids of the family estimated their break-up at 80 Ma, giving them insufficient time to shift orbits and impact Earth by 66 Ma.
Effects of impact

The collision would have released the same energy as 100 teratonnes of TNT (4.2×10 joules)—more than a billion times the energy of the atomic bombings of Hiroshima and Nagasaki. The Chicxulub impact caused a global catastrophe. Some of the phenomena were brief occurrences immediately following the impact, but there were also long-term geochemical and climatic disruptions that devastated the ecology.
The scientific consensus is that the asteroid impact at the K–Pg boundary left megatsunami deposits and sediments around the area of the Caribbean Sea and Gulf of Mexico, from the colossal waves created by the impact. These deposits have been identified in the La Popa basin in northeastern Mexico, platform carbonates in northeastern Brazil, in Atlantic deep-sea sediments, and in the form of the thickest-known layer of graded sand deposits, around 100 m (330 ft), in the Chicxulub crater itself, directly above the shocked granite ejecta. The megatsunami has been estimated at more than 100 m (330 ft) tall, as the asteroid fell into relatively shallow seas; in deep seas it would have been 4.6 km (2.9 mi) tall. Fossiliferous sedimentary rocks deposited during the K–Pg impact have been found in the Gulf of Mexico area, including tsunami wash deposits carrying remains of a mangrove-type ecosystem, indicating that water in the Gulf of Mexico sloshed back and forth repeatedly after the impact; dead fish left in these shallow waters were not disturbed by scavengers.
The re-entry of ejecta into Earth's atmosphere included a brief (hours-long) but intense pulse of infrared radiation, cooking exposed organisms. This is debated, with opponents arguing that local ferocious fires, probably limited to North America, fall short of global firestorms. This is the "Cretaceous–Paleogene firestorm debate". A paper in 2013 by a prominent modeler of nuclear winter suggested that, based on the amount of soot in the global debris layer, the entire terrestrial biosphere might have burned, implying a global soot-cloud blocking out the sun and creating an impact winter effect. If widespread fires occurred this would have exterminated the most vulnerable organisms that survived the period immediately after the impact. Experimental analysis suggests that any impact-induced wildfires were insufficient on their own to cause plant extinctions, and much of the thermal radiation generated by the impact would have been absorbed by the atmosphere and ejecta in the lower atmosphere.
Aside from the hypothesized fire effects on reduction of insolation, the impact would have created a humongous dust cloud that blocked sunlight for up to a year, inhibiting photosynthesis. The asteroid hit an area of gypsum and anhydrite rock containing a large amount of combustible hydrocarbons and sulfur, much of which was vaporized, thereby injecting sulfuric acid aerosols into the stratosphere, which might have reduced sunlight reaching the Earth's surface by more than 50%. Fine silicate dust also contributed to the intense impact winter, as did soot from wildfires. The climatic forcing of this impact winter was about 100 times more potent than that of the 1991 eruption of Mount Pinatubo. According to models of the Hell Creek Formation, the onset of global darkness would have reached its maximum in only a few weeks and likely lasted upwards of 2 years. Freezing temperatures probably lasted for at least three years. At Brazos section, the sea surface temperature dropped as much as 7 °C (13 °F) for decades after the impact. It would take at least ten years for such aerosols to dissipate, and would account for the extinction of plants and phytoplankton, and subsequently herbivores and their predators. Creatures whose food chains were based on detritus would have a reasonable chance of survival. In 2016, a scientific drilling project obtained deep rock-core samples from the peak ring around the Chicxulub impact crater. The discoveries confirmed that the rock comprising the peak ring had been shocked by immense pressure and melted in just minutes from its usual state into its present form. Unlike sea-floor deposits, the peak ring was made of granite originating much deeper in the earth, which had been ejected to the surface by the impact. Gypsum is a sulfate-containing rock usually present in the shallow seabed of the region; it had been almost entirely removed, vaporized into the atmosphere. The impactor was large enough to create a 190-kilometer-wide (120 mi) peak ring, to melt, shock, and eject deep granite, to create colossal water movements, and to eject an immense quantity of vaporized rock and sulfates into the atmosphere, where they would have persisted for several years. This worldwide dispersal of dust and sulfates would have affected climate catastrophically, led to large temperature drops, and devastated the food chain.
The release of large quantities of sulphur aerosols into the atmosphere as a consequence of the impact would also have caused acid rain. Oceans acidified as a result. This decrease in ocean pH would kill many organisms that grow shells of calcium carbonate. The heating of the atmosphere during the impact itself may have also generated nitric acid rain through the production of nitrogen oxides and their subsequent reaction with water vapour.
After the impact winter, the Earth entered a period of global warming as a result of the vapourisation of carbonates into carbon dioxide, whose long residence time in the atmosphere ensured significant warming would occur after more short-lived cooling gases dissipated. Carbon monoxide concentrations also increased and caused particularly devastating global warming because of the consequent increases in tropospheric ozone and methane concentrations. The impact's injection of water vapour into the atmosphere also produced major climatic perturbations.
The end-Cretaceous event is the only mass extinction definitively known to be associated with an impact, and other large extraterrestrial impacts, such as the Manicouagan Reservoir impact, do not coincide with any noticeable extinction events.

Multiple impact event
Other crater-like topographic features have also been proposed as impact craters formed in connection with Cretaceous–Paleogene extinction. This suggests the possibility of near-simultaneous multiple impacts, perhaps from a fragmented asteroidal object similar to the Shoemaker–Levy 9 impact with Jupiter. In addition to the 180 km (110 mi) Chicxulub crater, there is the 24 km (15 mi) Boltysh crater in Ukraine (65.17±0.64 Ma), the 20 km (12 mi) Silverpit crater in the North Sea (59.5±14.5 Ma) possibly formed by bolide impact, and the controversial and much larger 600 km (370 mi) Shiva crater. Any other craters that might have formed in the Tethys Ocean would since have been obscured by the northward tectonic drift of Africa and India.
Deccan Traps
The Deccan Traps, which erupted close to the boundary between the Mesozoic and Cenozoic, have been cited as an alternate explanation for the mass extinction. Before 2000, arguments that the Deccan Traps flood basalts caused the extinction were usually linked to the view that the extinction was gradual, as the flood basalt events were thought to have started around 68 Mya and lasted more than 2 million years. The most recent evidence shows that the traps erupted over a period of only 800,000 years spanning the K–Pg boundary, and therefore may be responsible for the extinction and the delayed biotic recovery thereafter.
The Deccan Traps could have caused extinction through several mechanisms, including the release of dust and sulfuric aerosols into the air, which might have blocked sunlight and thereby reduced photosynthesis in plants. In addition, the latest Cretaceous saw a rise in global temperatures; Deccan Traps volcanism resulted in carbon dioxide emissions that increased the greenhouse effect when the dust and aerosols cleared from the atmosphere. Plant fossils record a 250 ppm increase in carbon dioxide concentrations across the K-Pg boundary likely attributable to Deccan Traps activity. The increased carbon dioxide emissions also caused acid rain, evidenced by increased mercury deposition due to increased solubility of mercury compounds in more acidic water.
Evidence for extinctions caused by the Deccan Traps includes the reduction in diversity of marine life when the climate near the K–Pg boundary increased in temperature. The temperature increased about three to four degrees very rapidly between 65.4 and 65.2 million years ago, which is very near the time of the extinction event. Not only did the climate temperature increase, but the water temperature decreased, causing a drastic decrease in marine diversity. Evidence from Tunisia indicates that marine life was deleteriously affected by a major period of increased warmth and humidity linked to a pulse of intense Deccan Traps activity, and that marine extinctions there began before the impact event. Charophyte declines in the Songliao Basin, China before the asteroid impact have been concluded to be connected to climate changes caused by Deccan Traps activity.
In the years when the Deccan Traps hypothesis was linked to a slower extinction, Luis Alvarez (d. 1988) replied that paleontologists were being misled by sparse data. While his assertion was not initially well-received, later intensive field studies of fossil beds lent weight to his claim. Eventually, most paleontologists began to accept the idea that the mass extinctions at the end of the Cretaceous were largely or at least partly due to a massive Earth impact. Even Walter Alvarez acknowledged that other major changes might have contributed to the extinctions. More recent arguments against the Deccan Traps as an extinction cause include that the timeline of Deccan Traps activity and pulses of climate change has been found by some studies to be asynchronous, that palynological changes do not coincide with intervals of volcanism, and that many sites show climatic stability during the latest Maastrichtian and no sign of major disruptions caused by volcanism. Multiple modelling studies conclude that an impact event, not volcanism, fits best with available evidence of extinction patterns.
Combining these theories, some geophysical models suggest that the impact contributed to the Deccan Traps. These models, combined with high-precision radiometric dating, suggest that the Chicxulub impact could have triggered some of the largest Deccan eruptions, as well as eruptions at active volcano sites anywhere on Earth.
Maastrichtian sea-level regression
There is clear evidence that sea levels fell in the final stage of the Cretaceous by more than at any other time in the Mesozoic era. In some Maastrichtian stage rock layers from various parts of the world, the later layers are terrestrial; earlier layers represent shorelines and the earliest layers represent seabeds. These layers do not show the tilting and distortion associated with mountain building, therefore the likeliest explanation is a regression, a drop in sea level. There is no direct evidence for the cause of the regression, but the currently accepted explanation is that the mid-ocean ridges became less active and sank under their own weight.
A severe regression would have greatly reduced the continental shelf area, the most species-rich part of the sea, and therefore could have been enough to cause a marine mass extinction. This change would not have caused the extinction of the ammonites. The regression would also have caused climate changes, partly by disrupting winds and ocean currents and partly by reducing the Earth's albedo and increasing global temperatures. Marine regression also resulted in the loss of epeiric seas, such as the Western Interior Seaway of North America. The loss of these seas greatly altered habitats, removing coastal plains that ten million years before had been host to diverse communities such as are found in rocks of the Dinosaur Park Formation. Another consequence was an expansion of freshwater environments, since continental runoff now had longer distances to travel before reaching oceans. While this change was favorable to freshwater vertebrates, those that prefer marine environments, such as sharks, suffered.
However, sea level fall as a cause of the extinction event is contradicted by other evidence, namely that sections which show no sign of marine regression still show evidence of a major drop in diversity.
Multiple causes
Proponents of multiple causation view the suggested single causes as either too small to produce the vast scale of the extinction, or not likely to produce its observed taxonomic pattern. In a review article, J. David Archibald and David E. Fastovsky discussed a scenario combining three major postulated causes: volcanism, marine regression, and extraterrestrial impact. In this scenario, terrestrial and marine communities were stressed by the changes in, and loss of, habitats. Dinosaurs, as the largest vertebrates, were the first affected by environmental changes, and their diversity declined. At the same time, particulate materials from volcanism cooled and dried areas of the globe. Then an impact event occurred, causing collapses in photosynthesis-based food chains, both in the already-stressed terrestrial food chains and in the marine food chains.
Based on studies at Seymour Island in Antarctica, Sierra Petersen and colleagues argue that there were two separate extinction events near the Cretaceous–Paleogene boundary, with one correlating to Deccan Trap volcanism and one correlated with the Chicxulub impact. The team analyzed combined extinction patterns using a new clumped isotope temperature record from a hiatus-free, expanded K–Pg boundary section. They documented a 7.8±3.3 °C warming synchronous with the onset of Deccan Traps volcanism and a second, smaller warming at the time of meteorite impact. They suggested that local warming had been amplified due to the simultaneous disappearance of continental or sea ice. Intra-shell variability indicates a possible reduction in seasonality after Deccan eruptions began, continuing through the meteorite event. Species extinction at Seymour Island occurred in two pulses that coincide with the two observed warming events, directly linking the end-Cretaceous extinction at this site to both volcanic and meteorite events via climate change.

See also
- Climate across Cretaceous–Paleogene boundary
- Late Devonian extinction – One of the five most severe extinction events in the history of the Earth's biota
- List of possible impact structures on Earth
- Late Ordovician mass extinction – Extinction event around 444 million years ago
- Permian–Triassic extinction event – Earth's most severe extinction event
- Timeline of Cretaceous–Paleogene extinction event research – Research timeline
- Triassic–Jurassic extinction event – Mass extinction ending the Triassic period
Explanatory notes
- ^ The abbreviation is derived from the juxtaposition of K, the common abbreviation for the Cretaceous, which in turn originates from the correspondent German term Kreide; and Pg, which is the abbreviation for the Paleogene.
- ^ The former designation includes the term 'Tertiary' (abbreviated as T), which is now discouraged as a formal geochronological unit by the International Commission on Stratigraphy.
- ^ Shocked minerals have their internal structure deformed, and are created by intense pressures as in nuclear blasts and meteorite impacts.
Citations
- ^ Ogg, James G.; Gradstein, F. M.; Gradstein, Felix M. (2004). A geologic time scale 2004. Cambridge, UK: Cambridge University Press. ISBN 978-0-521-78142-8.
- ^ "International Chronostratigraphic Chart". stratigraphy.org. International Commission on Stratigraphy. 2015. Archived from the original on 30 May 2014. Retrieved 29 April 2015.
- ^ Fortey, Richard (1999). Life: A natural history of the first four billion years of life on Earth. Vintage. pp. 238–260. ISBN 978-0-375-70261-7.
- ^ Muench, David; Muench, Marc; Gilders, Michelle A. (2000). Primal Forces. Portland, Oregon: Graphic Arts Center Publishing. p. 20. ISBN 978-1-55868-522-2.
- ^ Jones, Heather L.; Westerhold, Thomas; Birch, Heather; et al. (18 January 2023). "Stratigraphy of the Cretaceous/Paleogene (K/Pg) boundary at the Global Stratotype Section and Point (GSSP) in El Kef, Tunisia: New insights from the El Kef Coring Project". Geological Society of America Bulletin. 135 (9–10): 2451. Bibcode:2023GSAB..135.2451J. doi:10.1130/B36487.1. S2CID 256021543.
- ^ Irizarry, Kayla M.; Witts, James T.; Garb, Matthew P.; et al. (15 January 2023). "Faunal and stratigraphic analysis of the basal Cretaceous-Paleogene (K-Pg) boundary event deposits, Brazos River, Texas, USA". Palaeogeography, Palaeoclimatology, Palaeoecology. 610: 111334. Bibcode:2023PPP...61011334I. doi:10.1016/j.palaeo.2022.111334. S2CID 254345541.
- ^ Ferreira da Silva, Luiza Carine; Santos, Alessandra; Fauth, Gerson; et al. (April 2023). "High-latitude Cretaceous–Paleogene transition: New paleoenvironmental and paleoclimatic insights from Seymour Island, Antarctica". Marine Micropaleontology. 180: 102214. Bibcode:2023MarMP.180j2214F. doi:10.1016/j.marmicro.2023.102214. S2CID 256834649.
- ^ Schulte, Peter; Alegret, L.; Arenillas, I.; et al. (5 March 2010). "The Chicxulub Asteroid Impact and Mass Extinction at the Cretaceous-Paleogene Boundary" (PDF). Science. 327 (5970): 1214–1218. Bibcode:2010Sci...327.1214S. doi:10.1126/science.1177265. PMID 20203042. S2CID 2659741.
- ^ Alvarez, Luis (10 March 1981). "The Asteroid and the Dinosaur (Nova S08E08, 1981)". IMDB. PBS-WGBH/Nova. Retrieved 12 June 2020.
- ^ Sleep, Norman H.; Lowe, Donald R. (9 April 2014). "Scientists reconstruct ancient impact that dwarfs dinosaur-extinction blast". agu.org (Press release). American Geophysical Union. Archived from the original on 1 January 2017. Retrieved 30 December 2016.
- ^ Amos, Jonathan (15 May 2017). "Dinosaur asteroid hit 'worst possible place'". BBC News Online. Archived from the original on 18 March 2018. Retrieved 16 March 2018.
- ^ Alvarez, Luis W.; Alvarez, Walter; Asaro, F.; Michel, H. V. (1980). "Extraterrestrial cause for the Cretaceous–Tertiary extinction" (PDF). Science. 208 (4448): 1095–1108. Bibcode:1980Sci...208.1095A. doi:10.1126/science.208.4448.1095. PMID 17783054. S2CID 16017767. Archived from the original (PDF) on 24 August 2019.
- ^ Vellekoop, J.; Sluijs, A.; Smit, J.; et al. (May 2014). "Rapid short-term cooling following the Chicxulub impact at the Cretaceous-Paleogene boundary". Proceedings of the National Academy of Sciences of the United States of America. 111 (21): 7537–7541. Bibcode:2014PNAS..111.7537V. doi:10.1073/pnas.1319253111. PMC 4040585. PMID 24821785.
- ^ Hildebrand, A. R.; Penfield, G. T.; Kring, David A.; et al. (1991). "Chicxulub crater: a possible Cretaceous/Tertiary boundary impact crater on the Yucatán peninsula, Mexico". Geology. 19 (9): 867–871. Bibcode:1991Geo....19..867H. doi:10.1130/0091-7613(1991)019<0867:ccapct>2.3.co;2.
- ^ Joel, Lucas (21 October 2019). "The dinosaur-killing asteroid acidified the ocean in a flash: the Chicxulub event was as damaging to life in the oceans as it was to creatures on land, a study shows". The New York Times. Archived from the original on 24 October 2019. Retrieved 24 October 2019.
- ^ Henehan, Michael J. (21 October 2019). "Rapid ocean acidification and protracted Earth system recovery followed the end-Cretaceous Chicxulub impact". Proceedings of the National Academy of Sciences of the United States of America. 116 (45): 22500–22504. Bibcode:2019PNAS..11622500H. doi:10.1073/pnas.1905989116. PMC 6842625. PMID 31636204.
- ^ Keller, Gerta (2012). "The Cretaceous–Tertiary mass extinction, Chicxulub impact, and Deccan volcanism. Earth and life". In Talent, John (ed.). Earth and Life: Global Biodiversity, Extinction Intervals and Biogeographic Perturbations Through Time. Springer. pp. 759–793. ISBN 978-90-481-3427-4.
- ^ Bosker, Bianca (September 2018). "The nastiest feud in science: A Princeton geologist has endured decades of ridicule for arguing that the fifth extinction was caused not by an asteroid but by a series of colossal volcanic eruptions. But she's reopened that debate". The Atlantic Monthly. Archived from the original on 21 February 2019. Retrieved 30 January 2019.
- ^ Joel, Lucas (16 January 2020). "Asteroid or Volcano? New Clues to the Dinosaurs' Demise". The New York Times. Retrieved 17 January 2020.
- ^ Hull, Pincelli M.; Bornemann, André; Penman, Donald E. (17 January 2020). "On impact and volcanism across the Cretaceous-Paleogene boundary". Science. 367 (6475): 266–272. Bibcode:2020Sci...367..266H. doi:10.1126/science.aay5055. hdl:20.500.11820/483a2e77-318f-476a-8fec-33a45fbdc90b. PMID 31949074. S2CID 210698721.
- ^ Chiarenza, Alfio Alessandro; Farnsworth, Alexander; Mannion, Philip D.; et al. (21 July 2020). "Asteroid impact, not volcanism, caused the end-Cretaceous dinosaur extinction". Proceedings of the National Academy of Sciences of the United States of America. 117 (29): 17084–17093. Bibcode:2020PNAS..11717084C. doi:10.1073/pnas.2006087117. ISSN 0027-8424. PMC 7382232. PMID 32601204.
- ^ Longrich, Nicholas R.; Tokaryk, Tim; Field, Daniel J. (2011). "Mass extinction of birds at the Cretaceous–Paleogene (K–Pg) boundary". Proceedings of the National Academy of Sciences of the United States of America. 108 (37): 15253–15257. Bibcode:2011PNAS..10815253L. doi:10.1073/pnas.1110395108. PMC 3174646. PMID 21914849.
- ^ Longrich, N. R.; Bhullar, B.-A. S.; Gauthier, J. A. (December 2012). "Mass extinction of lizards and snakes at the Cretaceous-Paleogene boundary". Proceedings of the National Academy of Sciences of the United States of America. 109 (52): 21396–401. Bibcode:2012PNAS..10921396L. doi:10.1073/pnas.1211526110. PMC 3535637. PMID 23236177.
- ^ Labandeira, C. C.; Johnson, K. R.; Lang, P. (2002). "Preliminary assessment of insect herbivory across the Cretaceous-Tertiary boundary: Major extinction and minimum rebound". In Hartman, J.H.; Johnson, K.R.; Nichols, D.J. (eds.). The Hell Creek formation and the Cretaceous-Tertiary boundary in the northern Great Plains: An integrated continental record of the end of the Cretaceous. Geological Society of America. pp. 297–327. ISBN 978-0-8137-2361-7.
- ^ Rehan, Sandra M.; Leys, Remko; Schwarz, Michael P. (2013). "First evidence for a massive extinction event affecting bees close to the K-T boundary". PLOS ONE. 8 (10): e76683. Bibcode:2013PLoSO...876683R. doi:10.1371/journal.pone.0076683. PMC 3806776. PMID 24194843.
- ^ Nichols, D. J.; Johnson, K. R. (2008). Plants and the K–T Boundary. Cambridge, UK: Cambridge University Press.
- ^ Friedman, M. (2009). "Ecomorphological selectivity among marine teleost fishes during the end-Cretaceous extinction". Proceedings of the National Academy of Sciences. 106 (13). Washington, DC: 5218–5223. Bibcode:2009PNAS..106.5218F. doi:10.1073/pnas.0808468106. PMC 2664034. PMID 19276106.
- ^ Jablonski, D.; Chaloner, W. G. (1994). "Extinctions in the fossil record (and discussion)". Philosophical Transactions of the Royal Society of London B. 344 (1307): 11–17. doi:10.1098/rstb.1994.0045.
- ^ Alroy, John (1999). "The fossil record of North American Mammals: evidence for a Palaeocene evolutionary radiation". Systematic Biology. 48 (1): 107–118. doi:10.1080/106351599260472. PMID 12078635.
- ^ Feduccia, Alan (1995). "Explosive evolution in Tertiary birds and mammals". Science. 267 (5198): 637–638. Bibcode:1995Sci...267..637F. doi:10.1126/science.267.5198.637. PMID 17745839. S2CID 42829066.
- ^ Friedman, M. (2010). "Explosive morphological diversification of spiny-finned teleost fishes in the aftermath of the end-Cretaceous extinction". Proceedings of the Royal Society B. 277 (1688): 1675–1683. doi:10.1098/rspb.2009.2177. PMC 2871855. PMID 20133356.
- ^ Weishampel, D. B.; Barrett, P. M. (2004). "Dinosaur distribution". In Weishampel, David B.; Dodson, Peter; Osmólska, Halszka (eds.). The Dinosauria (2nd ed.). Berkeley, CA: University of California Press. pp. 517–606. ISBN 978-0-520-24209-8. OCLC 441742117.
- ^ Barrera, Enriqueta; Keller, Gerta (1 October 1994). "Productivity across the Cretaceous/Tertiary boundary in high latitudes". Geological Society of America Bulletin. 106 (10): 1254–1266. doi:10.1130/0016-7606(1994)106<1254:PATCTB>2.3.CO;2.
- ^ Wilf, P.; Johnson, K.R. (2004). "Land plant extinction at the end of the Cretaceous: A quantitative analysis of the North Dakota megafloral record". Paleobiology. 30 (3): 347–368. doi:10.1666/0094-8373(2004)030<0347:LPEATE>2.0.CO;2. S2CID 33880578.
- ^ MacLeod, N.; Rawson, P.F.; Forey, P.L.; Banner, F.T.; Boudagher-Fadel, M.K.; Bown, P.R.; Burnett, J.A.; Chambers, P.; Culver, S.; Evans, S.E.; Jeffery, C.; Kaminski, M.A.; Lord, A.R.; Milner, A.C.; Milner, A.R.; Morris, N.; Owen, E.; Rosen, B.R.; Smith, A.B.; Taylor, P.D.; Urquhart, E.; Young, J.R. (1997). "The Cretaceous–Tertiary biotic transition". Journal of the Geological Society. 154 (2): 265–292. Bibcode:1997JGSoc.154..265M. doi:10.1144/gsjgs.154.2.0265. S2CID 129654916.
- ^ Sheehan, Peter M.; Hansen, Thor A. (1986). "Detritus feeding as a buffer to extinction at the end of the Cretaceous" (PDF). Geology. 14 (10): 868–870. Bibcode:1986Geo....14..868S. doi:10.1130/0091-7613(1986)14<868:DFAABT>2.0.CO;2. S2CID 54860261. Archived from the original (PDF) on 27 February 2019.
- ^ Aberhan, M.; Weidemeyer, S.; Kieesling, W.; Scasso, R.A.; Medina, F.A. (2007). "Faunal evidence for reduced productivity and uncoordinated recovery in Southern Hemisphere Cretaceous-Paleogene boundary sections". Geology. 35 (3): 227–230. Bibcode:2007Geo....35..227A. doi:10.1130/G23197A.1.
- ^ Sheehan, Peter M.; Fastovsky, D. E. (1992). "Major extinctions of land-dwelling vertebrates at the Cretaceous-Tertiary boundary, eastern Montana". Geology. 20 (6): 556–560. Bibcode:1992Geo....20..556S. doi:10.1130/0091-7613(1992)020<0556:MEOLDV>2.3.CO;2.
- ^ García-Girón, Jorge; Chiarenza, Alfio Alessandro; Alahuhta, Janne; DeMar, David G.; Heino, Jani; Mannion, Philip D.; Williamson, Thomas E.; Wilson Mantilla, Gregory P.; Brusatte, Stephen L. (9 December 2022). "Shifts in food webs and niche stability shaped survivorship and extinction at the end-Cretaceous". Science Advances. 8 (49): eadd5040. doi:10.1126/sciadv.add5040. PMC 9728968. PMID 36475805.
- ^ Kauffman, E. (2004). "Mosasaur predation on upper Cretaceous nautiloids and ammonites from the United States Pacific Coast" (PDF). PALAIOS. 19 (1): 96–100. Bibcode:2004Palai..19...96K. doi:10.1669/0883-1351(2004)019<0096:MPOUCN>2.0.CO;2. S2CID 130690035.
- ^ Clyde, William C.; Wilf, Peter; Iglesias, Ari; Slingerland, Rudy L.; Barnum, Timothy; Bijl, Peter K.; Bralower, Timothy J.; Brinkhuis, Henk; Comer, Emily E.; Huber, Brian T.; Ibañez-Mejia, Mauricio; Jicha, Brian R.; Krause, J. Marcelo; Schueth, Jonathan D.; Singer, Bradley S.; Raigemborn, María Sol; Schmitz, Mark D.; Sluijs, Appy; Zamaloa, María del Carmen (1 March 2014). "New age constraints for the Salamanca Formation and lower Río Chico Group in the western San Jorge Basin, Patagonia, Argentina: Implications for Cretaceous-Paleogene extinction recovery and land mammal age correlations". Geological Society of America Bulletin. 126 (3–4): 289–306. Bibcode:2014GSAB..126..289C. doi:10.1130/B30915.1. hdl:11336/80135. S2CID 129962470.
- ^ Pospichal, J. J. (1996). "Calcareous nannofossils and clastic sediments at the Cretaceous–Tertiary boundary, northeastern Mexico". Geology. 24 (3): 255–258. Bibcode:1996Geo....24..255P. doi:10.1130/0091-7613(1996)024<0255:CNACSA>2.3.CO;2.
- ^ Bown, P. (2005). "Selective calcareous nannoplankton survivorship at the Cretaceous–Tertiary boundary". Geology. 33 (8): 653–656. Bibcode:2005Geo....33..653B. doi:10.1130/G21566.1.
- ^ Bambach, R. K.; Knoll, A. H.; Wang, S. C. (2004). "Origination, extinction, and mass depletions of marine diversity" (PDF). Paleobiology. 30 (4): 522–542. doi:10.1666/0094-8373(2004)030<0522:OEAMDO>2.0.CO;2. S2CID 17279135.
- ^ Jiang, Shijun; Bralower, Timothy J.; Patzkowsky, Mark E.; Kump, Lee R.; Schueth, Jonathan D. (28 February 2010). "Geographic controls on nannoplankton extinction across the Cretaceous/Palaeogene boundary". Nature Geoscience. 3 (4): 280–285. doi:10.1038/ngeo775. ISSN 1752-0908. Retrieved 22 August 2024.
- ^ Schueth, Jonathan D.; Bralower, Timothy J.; Jiang, Shijun; Patzkowsky, Mark E. (September 2015). "The role of regional survivor incumbency in the evolutionary recovery of calcareous nannoplankton from the Cretaceous/Paleogene (K/Pg) mass extinction". Paleobiology. 41 (4): 661–679. doi:10.1017/pab.2015.28. ISSN 0094-8373.
- ^ Gedl, P. (2004). "Dinoflagellate cyst record of the deep-sea Cretaceous-Tertiary boundary at Uzgru, Carpathian Mountains, Czech Republic". Special Publications of the Geological Society of London. 230 (1): 257–273. Bibcode:2004GSLSP.230..257G. doi:10.1144/GSL.SP.2004.230.01.13. S2CID 128771186.
- ^ Tantawy, Abdel Aziz (2011). "Calcareous Nannofossils Across the Cretaceous–Tertiary Boundary at Brazos, Texas, U.S.A.: Extinction and Survivorship, Biostratigraphy, and Paleoecology". In Keller, Gerta; Adatte, Thierry (eds.). The End-Cretaceous Mass Extinction and the Chicxulub Impact in Texas. Society of Sedimentary Geology. pp. 157–178. doi:10.2110/sepmsp.100.157. ISBN 9781565763098.
- ^ MacLeod, N. (1998). "Impacts and marine invertebrate extinctions". Special Publications of the Geological Society of London. 140 (1): 217–246. Bibcode:1998GSLSP.140..217M. doi:10.1144/GSL.SP.1998.140.01.16. S2CID 129875020.
- ^ Courtillot, V. (1999). Evolutionary Catastrophes: The science of mass extinction. Cambridge, UK: Cambridge University Press. p. 2. ISBN 978-0-521-58392-3.
- ^ Hansen, T.; Farrand, R.B.; Montgomery, H.A.; Billman, H.G.; Blechschmidt, G. (September 1987). "Sedimentology and extinction patterns across the Cretaceous-Tertiary boundary interval in east Texas". Cretaceous Research. 8 (3): 229–252. doi:10.1016/0195-6671(87)90023-1.
- ^ Arenillas, I.; Arz, J. A.; Molina, E.; Dupuis, C. (2000). "An independent test of planktic foraminiferal turnover across the Cretaceous/Paleogene (K/P) boundary at El Kef, Tunisia: Catastrophic mass extinction and possible survivorship". Micropaleontology. 46 (1): 31–49. JSTOR 1486024.
- ^ Keller, Gerta (April 1993). "The Cretaceous-Tertiary boundary transition in the Antarctic Ocean and its global implications". Marine Micropaleontology. 21 (1–3): 1–45. doi:10.1016/0377-8398(93)90010-U.
- ^ Macleod, Norman; Keller, Gerta (1 November 1991). "How complete are Cretaceous /Tertiary boundary sections? A chronostratigraphic estimate based on graphic correlation". Geological Society of America Bulletin. 103 (11): 1439. doi:10.1130/0016-7606(1991)103<1439:HCACTB>2.3.CO;2. ISSN 0016-7606.
- ^ MacLeod, N. (1996). "Nature of the Cretaceous-Tertiary (K–T) planktonic foraminiferal record: Stratigraphic confidence intervals, Signor–Lipps effect, and patterns of survivorship". In MacLeod, N.; Keller, G. (eds.). Cretaceous–Tertiary Mass Extinctions: Biotic and environmental changes. W.W. Norton. pp. 85–138. ISBN 978-0-393-96657-2.
- ^ Keller, G.; Li, L.; MacLeod, N. (1 January 1996). "The Cretaceous/Tertiary boundary stratotype section at El Kef, Tunisia: how catastrophic was the mass extinction?". Palaeogeography, Palaeoclimatology, Palaeoecology. 119 (3): 221–254. doi:10.1016/0031-0182(95)00009-7. ISSN 0031-0182.
- ^ MacLeod, Norman; Keller, Gerta (Spring 1994). "Comparative biogeographic analysis of planktic foraminiferal survivorship across the Cretaceous/Tertiary (K/T) boundary". Paleobiology. 20 (2): 143–177. doi:10.1017/S0094837300012653. ISSN 0094-8373.
- ^ Schulte, Peter; Speijer, Robert; Mai, Hartmut; Kontny, Agnes (1 February 2006). "The Cretaceous–Paleogene (K–P) boundary at Brazos, Texas: Sequence stratigraphy, depositional events and the Chicxulub impact". Sedimentary Geology. 184 (1–2): 77–109. doi:10.1016/j.sedgeo.2005.09.021.
- ^ Galeotti, S.; Bellagamba, M.; Kaminski, M. A.; Montanari, A. (2002). "Deep-sea benthic foraminiferal recolonisation following a volcaniclastic event in the lower Campanian of the Scaglia Rossa Formation (Umbria-Marche Basin, central Italy)". Marine Micropaleontology. 44 (1–2): 57–76. Bibcode:2002MarMP..44...57G. doi:10.1016/s0377-8398(01)00037-8.
- ^ Kuhnt, W.; Collins, E. S. (1996). "8. Cretaceous to Paleogene benthic foraminifers from the Iberia abyssal plain". Proceedings of the Ocean Drilling Program, Scientific Results. Proceedings of the Ocean Drilling Program. 149: 203–216. doi:10.2973/odp.proc.sr.149.254.1996.
- ^ Coles, G. P.; Ayress, M. A.; Whatley, R. C. (1990). "A comparison of North Atlantic and 20 Pacific deep-sea Ostracoda". In Whatley, R. C.; Maybury, C. (eds.). Ostracoda and Global Events. Chapman & Hall. pp. 287–305. ISBN 978-0-442-31167-4.
- ^ Brouwers, E. M.; de Deckker, P. (1993). "Late Maastrichtian and Danian Ostracode Faunas from Northern Alaska: Reconstructions of Environment and Paleogeography". PALAIOS. 8 (2): 140–154. Bibcode:1993Palai...8..140B. doi:10.2307/3515168. JSTOR 3515168.
- ^ Martins, Maria João Fernandes; Hunt, Gene; Thompson, Carmi Milagros; Lockwood, Rowan; Swaddle, John P.; Puckett, T. Markham (26 August 2020). "Shifts in sexual dimorphism across a mass extinction in ostracods: implications for sexual selection as a factor in extinction risk". Proceedings of the Royal Society B: Biological Sciences. 287 (1933). doi:10.1098/rspb.2020.0730. ISSN 0962-8452. PMC 7482269. PMID 32811315.
- ^ Samuels-Fair, Maya; Martins, Maria João Fernandes; Lockwood, Rowan; Swaddle, John P.; Hunt, Gene (June 2022). "Temporal shifts in ostracode sexual dimorphism from the Late Cretaceous to the late Eocene of the U.S. Coastal Plain". Marine Micropaleontology. 174: 101959. doi:10.1016/j.marmicro.2020.101959. hdl:10400.1/18610.
- ^ Schweitzer, Carrie E.; Feldmann, Rodney M. (June 2023). "Selective extinction at the end-Cretaceous and appearance of the modern Decapoda". Journal of Crustacean Biology. 43 (2). doi:10.1093/jcbiol/ruad018. ISSN 0278-0372.
- ^ Hyžný, Matúš; Perrier, Vincent; Robin, Ninon; Martin, Jeremy E.; Sarr, Raphaël (January 2016). "Costacopluma (Decapoda: Brachyura: Retroplumidae) from the Maastrichtian and Paleocene of Senegal: A survivor of K/Pg events". Cretaceous Research. 57: 142–156. doi:10.1016/j.cretres.2015.08.010.
- ^ Vescsei, A.; Moussavian, E. (1997). "Paleocene reefs on the Maiella Platform margin, Italy: An example of the effects of the cretaceous/tertiary boundary events on reefs and carbonate platforms". Facies. 36 (1): 123–139. Bibcode:1997Faci...36..123V. doi:10.1007/BF02536880. S2CID 129296658.
- ^ Rosen, B. R.; Turnšek, D. (1989). Jell A; Pickett JW (eds.). "Extinction patterns and biogeography of scleractinian corals across the Cretaceous/Tertiary boundary". Memoir of the Association of Australasian Paleontology. Proceedings of the Fifth International Symposium on Fossil Cnidaria including Archaeocyatha and Spongiomorphs (8). Brisbane, Queensland: 355–370.
- ^ Raup, D. M.; Jablonski, D. (1993). "Geography of end-Cretaceous marine bivalve extinctions". Science. 260 (5110): 971–973. Bibcode:1993Sci...260..971R. doi:10.1126/science.11537491. PMID 11537491.
- ^ MacLeod, K. G. (1994). "Extinction of Inoceramid Bivalves in Maastrichtian Strata of the Bay of Biscay Region of France and Spain". Journal of Paleontology. 68 (5): 1048–1066. Bibcode:1994JPal...68.1048M. doi:10.1017/S0022336000026652. S2CID 132641572.
- ^ Marshall, C. R.; Ward, P. D. (1996). "Sudden and Gradual Molluscan Extinctions in the Latest Cretaceous of Western European Tethys". Science. 274 (5291): 1360–1363. Bibcode:1996Sci...274.1360M. doi:10.1126/science.274.5291.1360. PMID 8910273. S2CID 1837900.
- ^ Hansen, Thor A.; Farrell, Benjamin R.; Upshaw, Banks (Spring 1993). "The first 2 million years after the Cretaceous-Tertiary boundary in east Texas: rate and paleoecology of the molluscan recovery". Paleobiology. 19 (2): 251–265. doi:10.1017/S0094837300015906. ISSN 0094-8373.
- ^ Lockwood, Rowan (4 March 2003). "Abundance not linked to survival across the end-Cretaceous mass extinction: Patterns in North American bivalves". Proceedings of the National Academy of Sciences of the United States of America. 100 (5): 2478–2482. doi:10.1073/pnas.0535132100. ISSN 0027-8424. PMC 151366. PMID 12601147.
- ^ Lockwood, Rowan (Fall 2004). "The K/T event and infaunality: morphological and ecological patterns of extinction and recovery in veneroid bivalves". Paleobiology. 30 (4): 507–521. doi:10.1666/0094-8373(2004)030<0507:TTEAIM>2.0.CO;2. ISSN 0094-8373.
- ^ Ward, P. D.; Kennedy, W. J.; MacLeod, K. G.; Mount, J. F. (1991). "Ammonite and inoceramid bivalve extinction patterns in Cretaceous/Tertiary boundary sections of the Biscay region (southwestern France, northern Spain)". Geology. 19 (12): 1181–1184. Bibcode:1991Geo....19.1181W. doi:10.1130/0091-7613(1991)019<1181:AAIBEP>2.3.CO;2.
- ^ Harries, P. J.; Johnson, K. R.; Cobban, W. A.; Nichols, D.J. (2002). "Marine Cretaceous-Tertiary boundary section in southwestern South Dakota: Comment and reply". Geology. 30 (10): 954–955. Bibcode:2002Geo....30..954H. doi:10.1130/0091-7613(2002)030<0955:MCTBSI>2.0.CO;2.
- ^ Iba, Yasuhiro; Mutterlose, Jörg; Tanabe, Kazushige; Sano, Shin-ichi; Misaki, Akihiro; Terabe, Kazunobu (1 May 2011). "Belemnite extinction and the origin of modern cephalopods 35 m.y. prior to the Cretaceous−Paleogene event". Geology. 39 (5): 483–486. Bibcode:2011Geo....39..483I. doi:10.1130/G31724.1. ISSN 1943-2682. Retrieved 18 November 2023.
- ^ Neraudeau, Didier; Thierry, Jacques; Moreau, Pierre (1 January 1997). "Variation in echinoid biodiversity during the Cenomanian-early Turonian transgressive episode in Charentes (France)". Bulletin de la Société Géologique de France. 168 (1): 51–61.
- ^ Wiese, Frank; Schlüter, Nils; Zirkel, Jessica; Herrle, Jens O.; Friedrich, Oliver (9 August 2023). Carnevale, Giorgio (ed.). "A 104-Ma record of deep-sea Atelostomata (Holasterioda, Spatangoida, irregular echinoids) – a story of persistence, food availability and a big bang". PLOS ONE. 18 (8): e0288046. doi:10.1371/journal.pone.0288046. ISSN 1932-6203. PMC 10411753. PMID 37556403.
- ^ Labandeira, Conrad C.; Johnson, Kirk R.; Wilf, Peter (2002). "Impact of the terminal Cretaceous event on plant–insect associations". Proceedings of the National Academy of Sciences of the United States of America. 99 (4): 2061–2066. Bibcode:2002PNAS...99.2061L. doi:10.1073/pnas.042492999. PMC 122319. PMID 11854501.
- ^ Wilf, P.; Labandeira, C. C.; Johnson, K. R.; Ellis, B. (2006). "Decoupled plant and insect diversity after the end-Cretaceous extinction". Science. 313 (5790): 1112–1115. Bibcode:2006Sci...313.1112W. doi:10.1126/science.1129569. PMID 16931760. S2CID 52801127.
- ^ Wiest, Logan A.; Lukens, William E.; Peppe, Daniel J.; Driese, Steven G.; Tubbs, Jack (1 February 2018). "Terrestrial evidence for the Lilliput effect across the Cretaceous-Paleogene (K-Pg) boundary". Palaeogeography, Palaeoclimatology, Palaeoecology. 491: 161–169. Bibcode:2018PPP...491..161W. doi:10.1016/j.palaeo.2017.12.005. ISSN 0031-0182.
- ^ Grimaldi, David A. (2007). Evolution of the Insects. Cambridge Univ Pr (E). ISBN 978-0-511-12388-7.
- ^ Kriwet, Jürgen; Benton, Michael J. (2004). "Neoselachian (Chondrichthyes, Elasmobranchii) Diversity across the Cretaceous–Tertiary Boundary". Palaeogeography, Palaeoclimatology, Palaeoecology. 214 (3): 181–194. Bibcode:2004PPP...214..181K. doi:10.1016/j.palaeo.2004.02.049.
- ^ Patterson, C. (1993). "Osteichthyes: Teleostei". In Benton, M. J. (ed.). The Fossil Record. Vol. 2. Springer. pp. 621–656. ISBN 978-0-412-39380-8.
- ^ Noubhani, Abdelmajid (2010). "The Selachians' faunas of the Moroccan phosphate deposits and the K-T mass extinctions". Historical Biology. 22 (1–3): 71–77. Bibcode:2010HBio...22...71N. doi:10.1080/08912961003707349. S2CID 129579498.
- ^ Guinot, Guillaume; Condamine, Fabien L. (23 February 2023). "Global impact and selectivity of the Cretaceous-Paleogene mass extinction among sharks, skates, and rays" (PDF). Science. 379 (6634): 802–806. Bibcode:2023Sci...379..802G. doi:10.1126/science.abn2080. PMID 36821692. S2CID 257103123.
- ^ Zinsmeister, W. J. (1 May 1998). "Discovery of fish mortality horizon at the K–T boundary on Seymour Island: Re-evaluation of events at the end of the Cretaceous". Journal of Paleontology. 72 (3): 556–571. Bibcode:1998JPal...72..556Z. doi:10.1017/S0022336000024331. S2CID 132206016.
- ^ Cione, Alberto L.; Santillana, Sergio; Gouiric-Cavalli, Soledad; Acosta Hospitaleche, Carolina; Gelfo, Javier N.; López, Guillermo M.; Reguero, Marcelo (May 2018). "Before and after the K/Pg extinction in West Antarctica: New marine fish records from Marambio (Seymour) Island". Cretaceous Research. 85: 250–265. doi:10.1016/j.cretres.2018.01.004. hdl:11336/99687.
- ^ Robertson, D. S.; McKenna, M. C.; Toon, O. B.; et al. (2004). "Survival in the first hours of the Cenozoic" (PDF). Geological Society of America Bulletin. 116 (5–6): 760–768. Bibcode:2004GSAB..116..760R. doi:10.1130/B25402.1. S2CID 44010682. Archived from the original (PDF) on 7 May 2019.
- ^ Friedman, Matt; V. Andrews, James; Saad, Hadeel; El-Sayed, Sanaa (16 June 2023). "The Cretaceous–Paleogene transition in spiny-rayed fishes: surveying "Patterson's Gap" in the acanthomorph skeletal record André Dumont medalist lecture 2018". Geologica Belgica. doi:10.20341/gb.2023.002. ISSN 1374-8505. Retrieved 22 August 2024.
- ^ Alfaro, Michael E.; Faircloth, Brant C.; Harrington, Richard C.; Sorenson, Laurie; Friedman, Matt; Thacker, Christine E.; Oliveros, Carl H.; Černý, David; Near, Thomas J. (12 March 2018). "Explosive diversification of marine fishes at the Cretaceous–Palaeogene boundary". Nature Ecology & Evolution. 2 (4): 688–696. doi:10.1038/s41559-018-0494-6. ISSN 2397-334X. PMID 29531346. Retrieved 22 August 2024.
- ^ Archibald, J. D.; Bryant, L. J. (1990). "Differential Cretaceous–Tertiary extinction of nonmarine vertebrates; evidence from northeastern Montana". In Sharpton, V.L.; Ward, P.D. (eds.). Global Catastrophes in Earth History: an Interdisciplinary Conference on Impacts, Volcanism, and Mass Mortality. Special Paper. Vol. 247. Geological Society of America. pp. 549–562. doi:10.1130/spe247-p549. ISBN 978-0-8137-2247-4.
- ^ Estes, R. (1964). "Fossil vertebrates from the late Cretaceous Lance formation, eastern Wyoming". University of California Publications, Department of Geological Sciences. 49: 1–180.
- ^ Gardner, J. D. (2000). "Albanerpetontid amphibians from the upper Cretaceous (Campanian and Maastrichtian) of North America". Geodiversitas. 22 (3): 349–388.
- ^ Feng, Yan-Jie; Blackburn, David C.; Liang, Dan; Hillis, David M.; Wake, David B.; Cannatella, David C.; Zhang, Peng (18 July 2017). "Phylogenomics reveals rapid, simultaneous diversification of three major clades of Gondwanan frogs at the Cretaceous–Paleogene boundary". Proceedings of the National Academy of Sciences of the United States of America. 114 (29): E5864–E5870. doi:10.1073/pnas.1704632114. ISSN 0027-8424. PMC 5530686. PMID 28673970.
- ^ Evans, Susan E.; Klembara, Jozef (2005). "A choristoderan reptile (Reptilia: Diapsida) from the Lower Miocene of northwest Bohemia (Czech Republic)". Journal of Vertebrate Paleontology. 25 (1): 171–184. doi:10.1671/0272-4634(2005)025[0171:ACRRDF]2.0.CO;2. S2CID 84097919.
- ^ Matsumoto, Ryoko; Evans, Susan E. (November 2015). "Morphology and function of the palatal dentition in Choristodera". Journal of Anatomy. 228 (3): 414–429. doi:10.1111/joa.12414. PMC 5341546. PMID 26573112.
- ^ Novacek, M. J. (1999). "100 million years of land vertebrate evolution: The Cretaceous-early Tertiary transition". Annals of the Missouri Botanical Garden. 86 (2): 230–258. doi:10.2307/2666178. JSTOR 2666178.
- ^ Holroyd, Patricia A.; Wilson, Gregory P.; Hutchinson, J. Howard (2013). "Temporal changes within the latest Cretaceous and early Paleogene turtle faunas of northeastern Montana". In Wilson, Gregory P.; Clemens, William A.; Horner, John R.; Hartman, Joseph H. (eds.). Through the End of the Cretaceous in the Type Locality of the Hell Creek Formation in Montana and Adjacent Areas. Boulder, CO: Geological Society of America. pp. 299–312. ISBN 978-0-8137-2503-1.
- ^ Cleary, Terri J.; Benson, Roger B. J.; Holroyd, Patricia A.; Barrett, Paul M. (10 May 2020). Mannion, Philip (ed.). "Tracing the patterns of non-marine turtle richness from the Triassic to the Palaeogene: from origin to global spread". Palaeontology. 63 (5): 753–774. doi:10.1111/pala.12486. ISSN 0031-0239.
- ^ Pérez-García, Adán (30 January 2020). "Surviving the Cretaceous-Paleogene mass extinction event: A terrestrial stem turtle in the Cenozoic of Laurasia". Scientific Reports. 10 (1): 1489. doi:10.1038/s41598-020-58511-8. ISSN 2045-2322. PMC 6992736. PMID 32001765.
- ^ Apesteguía, Sebastián; Novas, Fernando E. (2003). "Large Cretaceous sphenodontian from Patagonia provides insight into lepidosaur evolution in Gondwana". Nature. 425 (6958): 609–612. Bibcode:2003Natur.425..609A. doi:10.1038/nature01995. PMID 14534584. S2CID 4425130.
- ^ Lutz, D. (2005). Tuatara: A living fossil. DIMI Press. ISBN 978-0-931625-43-5.
- ^ Apesteguía, Sebastián; Gómez, Raúl O.; Rougier, Guillermo W. (7 October 2014). "The youngest South American rhynchocephalian, a survivor of the K/Pg extinction". Proceedings of the Royal Society B: Biological Sciences. 281 (1792): 20140811. doi:10.1098/rspb.2014.0811. ISSN 0962-8452. PMC 4150314. PMID 25143041.
- ^ Herrera-Flores, Jorge A.; Stubbs, Thomas L.; Benton, Michael J. (March 2021). "Ecomorphological diversification of squamates in the Cretaceous". Royal Society Open Science. 8 (3). 201961. Bibcode:2021RSOS....801961H. doi:10.1098/rsos.201961. ISSN 2054-5703. PMC 8074880. PMID 33959350.
- ^ Xing, Lida; Niu, Kecheng; Evans, Susan E. (January 2023). "A new polyglyphanodontian lizard with a complete lower temporal bar from the Upper Cretaceous of southern China". Journal of Systematic Palaeontology. 21 (1). Bibcode:2023JSPal..2181494X. doi:10.1080/14772019.2023.2281494. ISSN 1477-2019.
- ^ Klein, Catherine G.; Pisani, Davide; Field, Daniel J.; Lakin, Rebecca; Wills, Matthew A.; Longrich, Nicholas R. (14 September 2021). "Evolution and dispersal of snakes across the Cretaceous-Paleogene mass extinction". Nature Communications. 12 (1): 5335. Bibcode:2021NatCo..12.5335K. doi:10.1038/s41467-021-25136-y. PMC 8440539. PMID 34521829.
- ^ Čerňanský, Andrej; Daza, Juan; Tabuce, Rodolphe; Saxton, Elizabeth; Vidalenc, Dominique (December 2023). "An early Eocene pan-gekkotan from France could represent an extra squamate group that survived the K-Pg extinction". Acta Palaeontologica Polonica. 68. doi:10.4202/app.01083.2023. Retrieved 22 August 2024.
- ^ Martin, Jeremy E.; Vincent, Peggy; Tacail, Théo; Khaldoune, Fatima; Jourani, Essaid; Bardet, Nathalie; Balter, Vincent (5 June 2017). "Calcium Isotopic Evidence for Vulnerable Marine Ecosystem Structure Prior to the K/Pg Extinction". Current Biology. 27 (11): 1641–1644.e2. doi:10.1016/j.cub.2017.04.043. ISSN 0960-9822. PMID 28552352.
- ^ D'Hondt, Steven (17 August 2005). "Consequences of the Cretaceous/Paleogene Mass Extinction for Marine Ecosystems". Annual Review of Ecology, Evolution, and Systematics. 36: 295–317. doi:10.1146/annurev.ecolsys.35.021103.105715. S2CID 86073650.
- ^ Chatterjee, S.; Small, B. J. (1989). "New plesiosaurs from the Upper Cretaceous of Antarctica". Geological Society of London. Special Publications. 47 (1): 197–215. Bibcode:1989GSLSP..47..197C. doi:10.1144/GSL.SP.1989.047.01.15. S2CID 140639013.
- ^ O'Keefe, F. R. (2001). "A cladistic analysis and taxonomic revision of the Plesiosauria (Reptilia: Sauropterygia)". Acta Zoologica Fennica. 213: 1–63.
- ^ O'Gorman, José P. (December 2022). "Polycotylidae (Sauropterygia, Plesiosauria) from the La Colonia Formation, Patagonia, Argentina: Phylogenetic affinities of Sulcusuchus erraini and the Late Cretaceous circum-pacific polycotylid diversity". Cretaceous Research. 140: 105339. Bibcode:2022CrRes.14005339O. doi:10.1016/j.cretres.2022.105339. S2CID 251749728.
- ^ Fischer, Valentin; Bardet, Nathalie; Benson, Roger B. J.; Arkhangelsky, Maxim S.; Friedman, Matt (2016). "Extinction of fish-shaped marine reptiles associated with reduced evolutionary rates and global environmental volatility". Nature Communications. 7 (1): 10825. Bibcode:2016NatCo...710825F. doi:10.1038/ncomms10825. PMC 4786747. PMID 26953824.
- ^ Brochu, C. A. (2004). "Calibration age and quartet divergence date estimation". Evolution. 58 (6): 1375–1382. doi:10.1554/03-509. PMID 15266985. S2CID 198156470.
- ^ Jouve, S.; Bardet, N.; Jalil, N.-E.; Suberbiola, X. P.; Bouya, B.; Amaghzaz, M. (2008). "The oldest African crocodylian: phylogeny, paleobiogeography, and differential survivorship of marine reptiles through the Cretaceous-Tertiary boundary" (PDF). Journal of Vertebrate Paleontology. 28 (2): 409–421. doi:10.1671/0272-4634(2008)28[409:TOACPP]2.0.CO;2. S2CID 86503283.
- ^ Aubier, Paul; Jouve, Stéphane; Schnyder, Johann; Cubo, Jorge (20 February 2023). Mannion, Philip (ed.). "Phylogenetic structure of the extinction and biotic factors explaining differential survival of terrestrial notosuchians at the Cretaceous–Palaeogene crisis". Palaeontology. 66 (1): 12638. Bibcode:2023Palgy..6612638A. doi:10.1111/pala.12638. ISSN 0031-0239.
- ^ Martin, Jeremy E.; Pochat-Cottilloux, Yohan; Laurent, Yves; Perrier, Vincent; Robert, Emmanuel; Antoine, Pierre-Olivier (28 October 2022). "Anatomy and phylogeny of an exceptionally large sebecid (Crocodylomorpha) from the middle Eocene of southern France". Journal of Vertebrate Paleontology. 42 (4). Bibcode:2022JVPal..42E3828M. doi:10.1080/02724634.2023.2193828. ISSN 0272-4634. S2CID 258361595.
- ^ Forêt, Tom; Aubier, Paul; Jouve, Stéphane; Cubo, Jorge (23 April 2024). "Biotic and abiotic factors and the phylogenetic structure of extinction in the evolution of Tethysuchia". Paleobiology. 50 (2): 285–307. doi:10.1017/pab.2024.5. ISSN 0094-8373.
- ^ Company, J.; Ruiz-Omeñaca, J. I.; Pereda Suberbiola, X. (1999). "A long-necked pterosaur (Pterodactyloidea, Azhdarchidae) from the upper Cretaceous of Valencia, Spain". Geologie en Mijnbouw. 78 (3): 319–333. doi:10.1023/A:1003851316054. S2CID 73638590.
- ^ Barrett, P. M.; Butler, R. J.; Edwards, N. P.; Milner, A. R. (2008). "Pterosaur distribution in time and space: an atlas" (PDF). Zitteliana. 28: 61–107. Archived (PDF) from the original on 6 August 2017. Retrieved 31 August 2015.
- ^ Slack, K. E.; Jones, C. M.; Ando, T.; Harrison, G. L.; Fordyce, R. E.; Arnason, U.; Penny, D. (2006). "Early Penguin Fossils, Plus Mitochondrial Genomes, Calibrate Avian Evolution". Molecular Biology and Evolution. 23 (6): 1144–1155. doi:10.1093/molbev/msj124. PMID 16533822.
- ^ Penny, D.; Phillips, M. J. (2004). "The rise of birds and mammals: Are microevolutionary processes sufficient for macroevolution?" (PDF). Trends in Ecology and Evolution. 19 (10): 516–522. doi:10.1016/j.tree.2004.07.015. PMID 16701316.
- ^ Butler, Richard J.; Barrett, Paul M.; Nowbath, Stephen; Upchurch, Paul (2009). "Estimating the effects of sampling biases on pterosaur diversity patterns: Implications for hypotheses of bird / pterosaur competitive replacement". Paleobiology. 35 (3): 432–446. Bibcode:2009Pbio...35..432B. doi:10.1666/0094-8373-35.3.432. S2CID 84324007.
- ^ Prondvai, E.; Bodor, E. R.; Ösi, A. (2014). "Does morphology reflect osteohistology-based ontogeny? A case study of Late Cretaceous pterosaur jaw symphyses from Hungary reveals hidden taxonomic diversity" (PDF). Paleobiology. 40 (2): 288–321. Bibcode:2014Pbio...40..288P. doi:10.1666/13030. S2CID 85673254.
- ^ Longrich, N. R.; Martill, D. M.; Andres, B. (2018). "Late Maastrichtian pterosaurs from North Africa and mass extinction of Pterosauria at the Cretaceous-Paleogene boundary". PLOS Biology. 16 (3): e2001663. doi:10.1371/journal.pbio.2001663. PMC 5849296. PMID 29534059.
- ^ David, Archibald; Fastovsky, David (2004). "Dinosaur extinction" (PDF). In Weishampel, David B.; Dodson, Peter; Osmólska, Halszka (eds.). The Dinosauria (2nd ed.). Berkeley: University of California Press. pp. 672–684. ISBN 978-0-520-24209-8.
- ^ Ocampo, A.; Vajda, V.; Buffetaut, E. (2006). "Unravelling the Cretaceous–Paleogene (K–T) turnover, evidence from flora, fauna and geology in biological processes associated with impact events". In Cockell, C.; Gilmour, I.; Koeberl, C. (eds.). Biological Processes Associated with Impact Events. SpringerLink. pp. 197–219. doi:10.1007/3-540-25736-5_9. ISBN 978-3-540-25735-6.
- ^ Buffetaut, Eric (18 November 2004). "Polar dinosaurs and the question of dinosaur extinction: a brief review". Palaeogeography, Palaeoclimatology, Palaeoecology. 214 (3): 225–231. doi:10.1016/j.palaeo.2004.02.050.
- ^ Fastovsky, David E.; Bercovici, Antoine (January 2016). "The Hell Creek Formation and its contribution to the Cretaceous–Paleogene extinction: A short primer". Cretaceous Research. 57: 368–390. Bibcode:2016CrRes..57..368F. doi:10.1016/j.cretres.2015.07.007.
- ^ Dodson, Peter (1996). The Horned Dinosaurs: A Natural History. Princeton, NJ: Princeton University Press. pp. 279–281. ISBN 978-0-691-05900-6.
- ^ Rieraa, V.; Marmib, J.; Omsa, O.; Gomez, B. (March 2010). "Orientated plant fragments revealing tidal palaeocurrents in the Fumanya mudflat (Maastrichtian, southern Pyrenees): Insights in palaeogeographic reconstructions". Palaeogeography, Palaeoclimatology, Palaeoecology. 288 (1–4): 82–92. Bibcode:2010PPP...288...82R. doi:10.1016/j.palaeo.2010.01.037.
- ^ le Loeuff, J. (2012). "Paleobiogeography and biodiversity of Late Maastrichtian dinosaurs: How many dinosaur species became extinct at the Cretaceous-Tertiary boundary?". Bulletin de la Société Géologique de France. 183 (6): 547–559. doi:10.2113/gssgfbull.183.6.547.
- ^ Ryan, M. J.; Russell, A. P.; Eberth, D. A.; Currie, P. J. (2001). "The taphonomy of a Centrosaurus (Ornithischia: Ceratopsidae) bone bed from the Dinosaur Park formation (Upper Campanian), Alberta, Canada, with comments on cranial ontogeny". PALAIOS. 16 (5): 482–506. Bibcode:2001Palai..16..482R. doi:10.1669/0883-1351(2001)016<0482:ttoaco>2.0.co;2. S2CID 130116586.
- ^ Sloan, R. E.; Rigby, K.; van Valen, L. M.; Gabriel, Diane (1986). "Gradual dinosaur extinction and simultaneous ungulate radiation in the Hell Creek formation". Science. 232 (4750): 629–633. Bibcode:1986Sci...232..629S. doi:10.1126/science.232.4750.629. PMID 17781415. S2CID 31638639.
- ^ Fassett, J. E.; Lucas, S. G.; Zielinski, R. A.; Budahn, J. R. (2001). Compelling new evidence for Paleocene dinosaurs in the Ojo Alamo Sandstone San Juan Basin, New Mexico and Colorado, USA (PDF). International Conference on Catastrophic Events and Mass Extinctions: Impacts and Beyond, 9–12 July 2000. Vol. 1053. Vienna, Austria. pp. 45–46. Bibcode:2001caev.conf.3139F. Archived (PDF) from the original on 5 June 2011. Retrieved 18 May 2007.
- ^ Sullivan, R. M. (2003). "No Paleocene dinosaurs in the San Juan Basin, New Mexico". Geological Society of America Abstracts with Programs. 35 (5): 15. Archived from the original on 8 April 2011. Retrieved 2 July 2007.
- ^ Hou, L.; Martin, M.; Zhou, Z.; Feduccia, A. (1996). "Early Adaptive Radiation of Birds: Evidence from Fossils from Northeastern China". Science. 274 (5290): 1164–1167. Bibcode:1996Sci...274.1164H. doi:10.1126/science.274.5290.1164. PMID 8895459. S2CID 30639866.
- ^ Clarke, J.A.; Tambussi, C.P.; Noriega, J.I.; Erickson, G.M.; Ketcham, R.A. (2005). "Definitive fossil evidence for the extant avian radiation in the Cretaceous" (PDF). Nature. 433 (7023): 305–308. Bibcode:2005Natur.433..305C. doi:10.1038/nature03150. hdl:11336/80763. PMID 15662422. S2CID 4354309.
- ^ "Primitive birds shared dinosaurs' fate". Science Daily. 20 September 2011. Archived from the original on 24 September 2011. Retrieved 20 September 2011.
- ^ Ericson, P. G.; Anderson, C. L.; Britton, T.; et al. (December 2006). "Diversification of Neoaves: integration of molecular sequence data and fossils". Biology Letters. 2 (4): 543–7. doi:10.1098/rsbl.2006.0523. PMC 1834003. PMID 17148284.
- ^ Mitchell, K.J.; Llamas, B.; Soubrier, J.; Rawlence, N. J.; Worthy, T. H.; Wood, J.; Lee, M. S. Y.; Cooper, A. (2014). "Ancient DNA reveals elephant birds and kiwi are sister taxa and clarifies ratite bird evolution". Science. 344 (6186): 989–900. Bibcode:2014Sci...344..898M. doi:10.1126/science.1251981. hdl:2328/35953. PMID 24855267. S2CID 206555952.
- ^ Yonezawa, Takahiro; Segawa, Takahiro; Mori, Hiroshi; Campos, Paula F.; Hongoh, Yuichi; Endo, Hideki; Akiyoshi, Ayumi; Kohno, Naoki; Nishida, Shin; Wu, Jiaqi; Jin, Haofei (2017). "Phylogenomics and Morphology of Extinct Paleognaths Reveal the Origin and Evolution of the Ratites". Current Biology. 27 (1): 68–77. doi:10.1016/j.cub.2016.10.029. PMID 27989673.
- ^ Bininda-Emonds, O. R.; Cardillo M.; Jones, K. E.; MacPhee, R. D.; Beck, R. M.; Grenyer, R.; Price, S. A.; Vos, R. A.; Gittleman, J. L.; Purvis, A. (2007). "The delayed rise of present-day mammals". Nature. 446 (7135): 507–512. Bibcode:2007Natur.446..507B. doi:10.1038/nature05634. PMID 17392779. S2CID 4314965.
- ^ Gelfo, J. N.; Pascual, R. (2001). "Peligrotherium tropicalis (Mammalia, Dryolestida) from the early Paleocene of Patagonia, a survival from a Mesozoic Gondwanan radiation" (PDF). Geodiversitas. 23: 369–379. Archived from the original (PDF) on 12 February 2012.
- ^ Goin, F. J.; Reguero, M. A.; Pascual, R.; von Koenigswald, W.; Woodburne, M. O.; Case, J. A.; Marenssi, S. A.; Vieytes, C.; Vizcaíno, S. F. (2006). "First gondwanatherian mammal from Antarctica". Geological Society, London. Special Publications. 258 (1): 135–144. Bibcode:2006GSLSP.258..135G. doi:10.1144/GSL.SP.2006.258.01.10. S2CID 129493664.
- ^ McKenna, M. C.; Bell, S. K. (1997). Classification of mammals: Above the species level. Columbia University Press. ISBN 978-0-231-11012-9.
- ^ Wood, D. Joseph (2010). The Extinction of the Multituberculates Outside North America: a Global Approach to Testing the Competition Model (M.S.). The Ohio State University. Archived from the original on 8 April 2015. Retrieved 3 April 2015.
- ^ Pires, Mathias M.; Rankin, Brian D.; Silvestro, Daniele; Quental, Tiago B. (2018). "Diversification dynamics of mammalian clades during the K–Pg mass extinction". Biology Letters. 14 (9): 20180458. doi:10.1098/rsbl.2018.0458. PMC 6170748. PMID 30258031.
- ^ Springer, Mark S.; Foley, Nicole M.; Brady, Peggy L.; Gatesy, John; Murphy, William J. (29 November 2019). "Evolutionary Models for the Diversification of Placental Mammals Across the KPg Boundary". Frontiers in Genetics. 10: 1241. doi:10.3389/fgene.2019.01241. ISSN 1664-8021. PMC 6896846. PMID 31850081.
- ^ Shupinski, Alex B.; Wagner, Peter J.; Smith, Felisa A.; Lyons, S. Kathleen (3 July 2024). "Unique functional diversity during early Cenozoic mammal radiation of North America". Proceedings of the Royal Society B: Biological Sciences. 291 (2026). doi:10.1098/rspb.2024.0778. ISSN 1471-2954. PMC 11286128. PMID 38955231.
- ^ Springer, M. S.; Murphy, W. J.; Eizirik, E.; O'Brien, S. J. (2003). "Placental mammal diversification and the Cretaceous–Tertiary boundary". Proceedings of the National Academy of Sciences of the United States of America. 100 (3): 1056–1061. Bibcode:2003PNAS..100.1056S. doi:10.1073/pnas.0334222100. PMC 298725. PMID 12552136.
- ^ Halliday, Thomas John Dixon; Upchurch, Paul; Goswami, Anjali (29 June 2016). "Eutherians experienced elevated evolutionary rates in the immediate aftermath of the Cretaceous–Palaeogene mass extinction". Proceedings of the Royal Society B: Biological Sciences. 283 (1833): 20153026. doi:10.1098/rspb.2015.3026. ISSN 0962-8452. PMC 4936024. PMID 27358361.
- ^ Alroy, J. (May 1998). "Cope's rule and the dynamics of body mass evolution in North American fossil mammals" (PDF). Science. 280 (5364): 731–4. Bibcode:1998Sci...280..731A. doi:10.1126/science.280.5364.731. PMID 9563948.
- ^ How life blossomed after the dinosaurs died | Science | AAAS
- ^ "Mammals' bodies outpaced their brains right after the dinosaurs died". Science News. 31 March 2022. Retrieved 14 May 2022.
- ^ Bertrand, Ornella C.; Shelley, Sarah L.; Williamson, Thomas E.; Wible, John R.; Chester, Stephen G. B.; Flynn, John J.; Holbrook, Luke T.; Lyson, Tyler R.; Meng, Jin; Miller, Ian M.; Püschel, Hans P.; Smith, Thierry; Spaulding, Michelle; Tseng, Z. Jack; Brusatte, Stephen L. (April 2022). "Brawn before brains in placental mammals after the end-Cretaceous extinction" (PDF). Science. 376 (6588): 80–85. Bibcode:2022Sci...376...80B. doi:10.1126/science.abl5584. hdl:20.500.11820/d7fb8c6e-886e-4c1d-9977-0cd6406fda20. ISSN 0036-8075. PMID 35357913. S2CID 247853831.
- ^ Vajda, Vivi; Raine, J. Ian; Hollis, Christopher J. (2001). "Indication of global deforestation at the Cretaceous–Tertiary boundary by New Zealand fern spike". Science. 294 (5547): 1700–1702. Bibcode:2001Sci...294.1700V. doi:10.1126/science.1064706. PMID 11721051. S2CID 40364945.
- ^ Johnson, K.R.; Hickey, L.J. (1991). "Megafloral change across the Cretaceous Tertiary boundary in the northern Great Plains and Rocky Mountains". In Sharpton, V.I.; Ward, P.D. (eds.). Global Catastrophes in Earth History: An interdisciplinary conference on impacts, volcanism, and mass mortality. Geological Society of America. ISBN 978-0-8137-2247-4.
- ^ Askin, R.A.; Jacobson, S.R. (1996). "Palynological change across the Cretaceous–Tertiary boundary on Seymour Island, Antarctica: environmental and depositional factors". In Keller, G.; MacLeod, N. (eds.). Cretaceous–Tertiary Mass Extinctions: Biotic and Environmental Changes. W W Norton. ISBN 978-0-393-96657-2.
- ^ Wappler, Torsten; Currano, Ellen D.; Wilf, Peter; Rust, Jes; Labandeira, Conrad C. (22 December 2009). "No post-Cretaceous ecosystem depression in European forests? Rich insect-feeding damage on diverse middle Palaeocene plants, Menat, France". Proceedings of the Royal Society B: Biological Sciences. 276 (1677): 4271–4277. doi:10.1098/rspb.2009.1255. ISSN 0962-8452. PMC 2817104. PMID 19776074.
- ^ Herman, A. B.; Akhmetiev, M. A.; Kodrul, T. M.; Moiseeva, M. G.; Iakovleva, A. I. (24 February 2009). "Flora development in Northeastern Asia and Northern Alaska during the Cretaceous-Paleogene transitional epoch". Stratigraphy and Geological Correlation. 17 (1): 79–97. doi:10.1134/S0869593809010079. ISSN 0869-5938.
- ^ Herman, A. B. (10 December 2013). "Albian-Paleocene flora of the north pacific: Systematic composition, palaeofloristics and phytostratigraphy". Stratigraphy and Geological Correlation. 21 (7): 689–747. doi:10.1134/S0869593813070034. ISSN 0869-5938.
- ^ Bajdek, Piotr (10 May 2019). "Divergence rates of subviral pathogens of angiosperms abruptly decreased at the Cretaceous-Paleogene boundary". Rethinking Ecology. 4: 89–101. doi:10.3897/rethinkingecology.4.33014. S2CID 196664424. Retrieved 23 March 2023.
- ^ Thompson, Jamie B.; Ramírez-Barahona, Santiago (September 2023). "No phylogenetic evidence for angiosperm mass extinction at the Cretaceous–Palaeogene (K-Pg) boundary". Biology Letters. 19 (9). doi:10.1098/rsbl.2023.0314. ISSN 1744-957X. PMC 10498348. PMID 37700701.
- ^ Vajda, Vivi; McLoughlin, Stephen (5 March 2004). "Fungal Proliferation at the Cretaceous-Tertiary Boundary". Science. 303 (5663): 1489. doi:10.1126/science.1093807. PMID 15001770. S2CID 44720346.
- ^ Schultz, P.; d'Hondt, S. (1996). "Cretaceous–Tertiary (Chicxulub) impact angle and its consequences". Geology. 24 (11): 963–967. Bibcode:1996Geo....24..963S. doi:10.1130/0091-7613(1996)024<0963:CTCIAA>2.3.CO;2.
- ^ Field, Daniel J.; Bercovici, Antoine; Berv, Jacob S.; Dunn, Regan; Fastovsky, David E.; Lyson, Tyler R.; et al. (24 May 2018). "Early evolution of modern birds structured by global forest collapse at the end-Cretaceous mass extinction". Current Biology. 28 (11): 1825–1831.e2. Bibcode:2018CBio...28E1825F. doi:10.1016/j.cub.2018.04.062. PMID 29804807. S2CID 44075214.
- ^ "Online guide to the continental Cretaceous–Tertiary boundary in the Raton basin, Colorado and New Mexico". U.S. Geological Survey. 2004. Archived from the original on 25 September 2006. Retrieved 8 July 2007.
- ^ Smathers, G.A.; Mueller-Dombois, D. (1974). Invasion and Recovery of Vegetation after a Volcanic Eruption in Hawaii. Scientific Monograph. Vol. 5. United States National Park Service. Archived from the original on 3 April 2014. Retrieved 9 July 2007.
- ^ Fawcett, Jeffrey A.; Maere, Steven; Van de Peer, Yves (April 2009). "Plants with double genomes might have had a better chance to survive the Cretaceous-Tertiary extinction event". Proceedings of the National Academy of Sciences of the United States of America. 106 (14): 5737–5742. Bibcode:2009PNAS..106.5737F. doi:10.1073/pnas.0900906106. PMC 2667025. PMID 19325131.
- ^ "Dinosaur-killing asteroid strike gave rise to Amazon rainforest". BBC News. 2 April 2021. Retrieved 9 May 2021.
- ^ Carvalho, Mónica R.; Jaramillo, Carlos; Parra, Felipe de la; et al. (2 April 2021). "Extinction at the end-Cretaceous and the origin of modern Neotropical rainforests". Science. 372 (6537): 63–68. Bibcode:2021Sci...372...63C. doi:10.1126/science.abf1969. ISSN 0036-8075. PMID 33795451. S2CID 232484243. Retrieved 9 May 2021.
- ^ Visscher, H.; Brinkhuis, H.; Dilcher, D. L.; Elsik, W. C.; Eshet, Y.; Looy, C. V.; Rampino, M. R.; Traverse, A. (5 March 1996). "The terminal Paleozoic fungal event: evidence of terrestrial ecosystem destabilization and collapse". Proceedings of the National Academy of Sciences of the United States of America. 93 (5): 2155–2158. Bibcode:1996PNAS...93.2155V. doi:10.1073/pnas.93.5.2155. PMC 39926. PMID 11607638.
- ^ Jack Wolfe (1991). "Palaeobotanical evidence for a June 'impact winter' at the Cretaceous/Tertiary boundary". Nature. 352 (6334): 420. Bibcode:1991Natur.352..420W. doi:10.1038/352420a0. S2CID 4242454.
- ^ During, Melanie A. D.; Smit, Jan; Voeten, Dennis F. A. E.; Berruyer, Camille; Tafforeau, Paul; Sanchez, Sophie; Stein, Koen H. W.; Verdegaal-Warmerdam, Suzan J. A.; Van Der Lubbe, Jeroen H. J. L. (23 February 2022). "The Mesozoic terminated in boreal spring". Nature. 603 (7899): 91–94. Bibcode:2022Natur.603...91D. doi:10.1038/s41586-022-04446-1. PMC 8891016. PMID 35197634.
- ^ Depalma, Robert A.; Oleinik, Anton A.; Gurche, Loren P.; Burnham, David A.; Klingler, Jeremy J.; McKinney, Curtis J.; Cichocki, Frederick P.; Larson, Peter L.; Egerton, Victoria M.; Wogelius, Roy A.; Edwards, Nicholas P.; Bergmann, Uwe; Manning, Phillip L. (8 December 2021). "Seasonal calibration of the end-cretaceous Chicxulub impact event". Nature. 11 (1): 23704. Bibcode:2021NatSR..1123704D. doi:10.1038/s41598-021-03232-9. PMC 8655067. PMID 34880389.
- ^ During, Melanie A. D.; Smit, Jan; Voeten, Dennis F. A. E.; et al. (23 February 2022). "The Mesozoic terminated in boreal spring". Nature. 603 (7899): 91–94. Bibcode:2022Natur.603...91D. doi:10.1038/s41586-022-04446-1. PMC 8891016. PMID 35197634.
- ^ "Springtime was the season the dinosaurs died, ancient fish fossils suggest". Science. 23 February 2022. Retrieved 24 February 2022.
- ^ Barras, Colin (23 February 2022). "Fossil fish reveal timing of asteroid that killed the dinosaurs". Nature. 603 (7899): 17. Bibcode:2022Natur.603...17B. doi:10.1038/d41586-022-00511-x. PMID 35197589. S2CID 247083600.
- ^ Ouellette, Jennifer (23 February 2022). "An asteroid killed dinosaurs in spring—which might explain why mammals survived – New study sheds light on why species extinction was so selective after the K-Pg impact". Ars Technica. Retrieved 26 February 2022.
- ^ Signor, Philip W. III; Lipps, Jere H. (1982). "Sampling bias, gradual extinction patterns, and catastrophes in the fossil record". In Silver, L.T.; Schultz, Peter H. (eds.). Geological implications of impacts of large asteroids and comets on the Earth. Boulder, CO: Geological Society of America. pp. 291–296. ISBN 978-0-8137-2190-3. OCLC 4434434112. Special Publication 190. Retrieved 25 October 2015.
- ^ Mukhopadhyay, Sujoy (2001). "A Short Duration of the Cretaceous-Tertiary Boundary Event: Evidence from Extraterrestrial Helium-3" (PDF). Science. 291 (5510): 1952–1955. Bibcode:2001Sci...291.1952M. doi:10.1126/science.291.5510.1952. PMID 11239153.
- ^ Clyde, William C.; Ramezani, Jahandar; Johnson, Kirk R.; Bowring, Samuel A.; Jones, Matthew M. (15 October 2016). "Direct high-precision U–Pb geochronology of the end-Cretaceous extinction and calibration of Paleocene astronomical timescales". Earth and Planetary Science Letters. 452: 272–280. Bibcode:2016E&PSL.452..272C. doi:10.1016/j.epsl.2016.07.041.
- ^ Renne, Paul R.; Deino, Alan L.; Hilgen, Frederik J.; Kuiper, Klaudia F.; Mark, Darren F.; Mitchell, William S.; Morgan, Leah E.; Mundil, Roland; Smit, Jan (8 February 2013). "Time Scales of Critical Events Around the Cretaceous-Paleogene Boundary". Science. 339 (6120): 684–687. Bibcode:2013Sci...339..684R. doi:10.1126/science.1230492. ISSN 0036-8075. PMID 23393261.
- ^ Weisberger, Mindy (22 December 2021). "Darkness caused by dino-killing asteroid snuffed out life on Earth in 9 months". livescience.com. Retrieved 17 November 2022.
- ^ de Laubenfels, M. W. (1956). "Dinosaur extinction: One more hypothesis". Journal of Paleontology. 30 (1): 207–218. JSTOR 1300393.
- ^ Smit, J.; Klaver, J. (1981). "Sanidine spherules at the Cretaceous-Tertiary boundary indicate a large impact event". Nature. 292 (5818): 47–49. Bibcode:1981Natur.292...47S. doi:10.1038/292047a0. S2CID 4331801.
- ^ Olsson, Richard K.; Miller, Kenneth G.; Browning, James V.; Habib, Daniel; Sugarman, Peter J. (1 August 1997). "Ejecta layer at the Cretaceous-Tertiary boundary, Bass River, New Jersey (Ocean Drilling Program Leg 174AX)". Geology. 25 (8): 759. doi:10.1130/0091-7613(1997)025<0759:ELATCT>2.3.CO;2. ISSN 0091-7613.
- ^ Bohor, B. F.; Foord, E. E.; Modreski, P. J.; Triplehorn, D. M. (1984). "Mineralogic evidence for an impact event at the Cretaceous-Tertiary boundary". Science. 224 (4651): 867–9. Bibcode:1984Sci...224..867B. doi:10.1126/science.224.4651.867. PMID 17743194. S2CID 25887801.
- ^ Bohor, B. F.; Modreski, P. J.; Foord, E. E. (1987). "Shocked quartz in the Cretaceous-Tertiary boundary clays: Evidence for a global distribution". Science. 236 (4802): 705–709. Bibcode:1987Sci...236..705B. doi:10.1126/science.236.4802.705. PMID 17748309. S2CID 31383614.
- ^ Bourgeois, J.; Hansen, T. A.; Wiberg, P. A.; Kauffman, E. G. (1988). "A tsunami deposit at the Cretaceous-Tertiary boundary in Texas". Science. 241 (4865): 567–570. Bibcode:1988Sci...241..567B. doi:10.1126/science.241.4865.567. PMID 17774578. S2CID 7447635.
- ^ Bralower, Timothy J.; Paull, Charles K.; Mark Leckie, R. (1 April 1998). "The Cretaceous-Tertiary boundary cocktail: Chicxulub impact triggers margin collapse and extensive sediment gravity flows". Geology. 26 (4): 331. doi:10.1130/0091-7613(1998)026<0331:TCTBCC>2.3.CO;2. ISSN 0091-7613.
- ^ Pope, K. O.; Ocampo, A. C.; Kinsland, G. L.; Smith, R. (1996). "Surface expression of the Chicxulub crater". Geology. 24 (6): 527–530. Bibcode:1996Geo....24..527P. doi:10.1130/0091-7613(1996)024<0527:SEOTCC>2.3.CO;2. PMID 11539331.
- ^ Stöffler, Dieter; Artemieva, Natalya A.; Ivanov, Boris A.; Hecht, Lutz; Kenkmann, Thomas; Schmitt, Ralf Thomas; Tagle, Roald Alberto; Wittmann, Axel (26 January 2010). "Origin and emplacement of the impact formations at Chicxulub, Mexico, as revealed by the ICDP deep drilling at Yaxcopoil-1 and by numerical modeling". Meteoritics & Planetary Science. 39 (7): 1035–1067. doi:10.1111/j.1945-5100.2004.tb01128.x. ISSN 1086-9379.
- ^ Depalma, Robert A.; Smit, Jan; Burnham, David A.; Kuiper, Klaudia; Manning, Phillip L.; Oleinik, Anton; Larson, Peter; Maurrasse, Florentin J.; Vellekoop, Johan; Richards, Mark A.; Gurche, Loren; Alvarez, Walter (2019). "A seismically induced onshore surge deposit at the KPG boundary, North Dakota". Proceedings of the National Academy of Sciences of the United States of America. 116 (17): 8190–8199. Bibcode:2019PNAS..116.8190D. doi:10.1073/pnas.1817407116. PMC 6486721. PMID 30936306.
- ^ "National Natural Landmarks – National Natural Landmarks (U.S. National Park Service)". www.nps.gov. Retrieved 22 March 2019.
Year designated: 1966
- ^ Smit, J., et al. (2017) Tanis, a mixed marine-continental event deposit at the KPG Boundary in North Dakota caused by a seiche triggered by seismic waves of the Chicxulub Impact Paper No. 113–15, presented 23 October 2017 at the GSA Annual Meeting, Seattle, Washington, USA.
- ^ DePalma, R. et al. (2017) Life after impact: A remarkable mammal burrow from the Chicxulub aftermath in the Hell Creek Formation, North Dakota Paper No. 113–16, presented 23 October 2017 at the GSA Annual Meeting, Seattle, Washington, USA.
- ^ Kaskes, P.; Goderis, S.; Belza, J.; Tack, P.; DePalma, R. A.; Smit, J.; Vincze, Laszlo; Vabgaecje, F.; Claeys, P. (2019). "Caught in amber: Geochemistry and petrography of uniquely preserved Chicxulub microtektites from the Tanis K-Pg site from North Dakota (USA)". Large Meteorite Impacts VI 2019 (LPI Contrib. No. 2136) (PDF). Vol. 6. Houston, TX: Lunar and Planetary Institute. pp. 1–2. Retrieved 11 April 2021.
- ^ Barras, Colin (5 April 2019). "Does fossil site record dino-killing impact?". Science. 364 (6435): 10–11. Bibcode:2019Sci...364...10B. doi:10.1126/science.364.6435.10. PMID 30948530. S2CID 96434764.
- ^ Alegret, Laia; Molina, Eustoquio; Thomas, Ellen (July 2003). "Benthic foraminiferal turnover across the Cretaceous/Paleogene boundary at Agost (southeastern Spain): paleoenvironmental inferences". Marine Micropaleontology. 48 (3–4): 251–279. Bibcode:2003MarMP..48..251A. doi:10.1016/S0377-8398(03)00022-7.
- ^ Keller, G.; Adatte, T.; Stinnesbeck, W.; Rebolledo-Vieyra, M.; Fucugauchi, J. U.; Kramar, U.; Stüben, D. (2004). "Chicxulub impact predates the K–T boundary mass extinction". Proceedings of the National Academy of Sciences of the United States of America. 101 (11): 3753–3758. Bibcode:2004PNAS..101.3753K. doi:10.1073/pnas.0400396101. PMC 374316. PMID 15004276.
- ^ Keller, Gerta; Adatte, Thierry; Stinnesbeck, Wolfgang; STüBEN, Doris; Berner, Zsolt; Kramar, Utz; Harting, Markus (26 January 2010). "More evidence that the Chicxulub impact predates the K/T mass extinction". Meteoritics & Planetary Science. 39 (7): 1127–1144. doi:10.1111/j.1945-5100.2004.tb01133.x. ISSN 1086-9379.
- ^ Perlman, David. "Dinosaur extinction battle flares". sfgate.com. Archived from the original on 8 February 2013. Retrieved 8 February 2013.
- ^ Timms, Nicholas E.; Kirkland, Christopher L.; Cavosie, Aaron J.; Rae, Auriol S.P.; Rickard, William D.A.; Evans, Noreen J.; Erickson, Timmons M.; Wittmann, Axel; Ferrière, Ludovic; Collins, Gareth S.; Gulick, Sean P.S. (15 July 2020). "Shocked titanite records Chicxulub hydrothermal alteration and impact age". Geochimica et Cosmochimica Acta. 281: 12–30. doi:10.1016/j.gca.2020.04.031.
- ^ Bottke, W. F.; Vokrouhlický, D.; Nesvorný, D. (September 2007). "An asteroid breakup 160 Myr ago as the probable source of the K/T impactor". Nature. 449 (7158): 48–53. Bibcode:2007Natur.449...48B. doi:10.1038/nature06070. PMID 17805288. S2CID 4322622.
- ^ Majaess, D. J.; Higgins, D.; Molnar, L. A.; Haegert, M. J.; Lane, D. J.; Turner, D. G.; Nielsen, I. (February 2009). "New constraints on the asteroid 298 Baptistina, the alleged family member of the K/T impactor". The Journal of the Royal Astronomical Society of Canada. 103 (1): 7–10. arXiv:0811.0171. Bibcode:2009JRASC.103....7M.
- ^ Reddy, V.; Emery, J. P.; Gaffey, M. J.; Bottke, W. F.; Cramer, A.; Kelley, M. S. (December 2009). "Composition of 298 Baptistina: Implications for the K/T impactor link". Meteoritics & Planetary Science. 44 (12): 1917–1927. Bibcode:2009M&PS...44.1917R. CiteSeerX 10.1.1.712.8165. doi:10.1111/j.1945-5100.2009.tb02001.x. S2CID 39644763.
- ^ "NASA's WISE raises doubt about asteroid family believed responsible for dinosaur extinction". ScienceDaily. 20 September 2011. Archived from the original on 23 September 2011. Retrieved 21 September 2011.
- ^ Osterloff, Emily (2018). "How an asteroid ended the age of the dinosaurs". London: Natural History Museum. Archived from the original on 26 April 2022. Retrieved 18 May 2022.
- ^ Robertson, D. S.; Lewis, W. M.; Sheehan, P. M.; Toon, O. B. (2013). "K/Pg extinction: Re-evaluation of the heat/fire hypothesis". Journal of Geophysical Research. 118 (1): 329–336. Bibcode:2013JGRG..118..329R. doi:10.1002/jgrg.20018.
- ^ Pope, K. O.; d'Hondt, S. L.; Marshall. C. R. (1998). "Meteorite impact and the mass extinction of species at the Cretaceous/Tertiary boundary". Proceedings of the National Academy of Sciences of the United States of America. 95 (19): 11028–11029. Bibcode:1998PNAS...9511028P. doi:10.1073/pnas.95.19.11028. PMC 33889. PMID 9736679.
- ^ Brugger, Julia; Feulner, Georg; Petri, Stefan (2016). "Baby, it's cold outside: Climate model simulations of the effects of the asteroid impact at the end of the Cretaceous" (PDF). Geophysical Research Letters. 44 (1): 419–427. Bibcode:2017GeoRL..44..419B. doi:10.1002/2016GL072241. S2CID 53631053.
- ^ Bourgeois, J. (2009). "Chapter 3. Geologic effects and records of tsunamis" (PDF). In Robinson, A.R.; Bernard, E.N. (eds.). Tsunamis. The Sea (Ideas and Observations on Progress in the Study of the Seas). Vol. 15. Boston, MA: Harvard University. ISBN 978-0-674-03173-9. Retrieved 29 March 2012.
- ^ Lawton, T. F.; Shipley, K. W.; Aschoff, J. L.; Giles, K. A.; Vega, F. J. (2005). "Basinward transport of Chicxulub ejecta by tsunami-induced backflow, La Popa basin, northeastern Mexico, and its implications for distribution of impact-related deposits flanking the Gulf of Mexico". Geology. 33 (2): 81–84. Bibcode:2005Geo....33...81L. doi:10.1130/G21057.1.
- ^ Albertão, G. A.; P. P. Martins Jr. (1996). "A possible tsunami deposit at the Cretaceous-Tertiary boundary in Pernambuco, northeastern Brazil". Sed. Geol. 104 (1–4): 189–201. Bibcode:1996SedG..104..189A. doi:10.1016/0037-0738(95)00128-X.
- ^ Norris, R. D.; Firth, J.; Blusztajn, J. S. & Ravizza, G. (2000). "Mass failure of the North Atlantic margin triggered by the Cretaceous-Paleogene bolide impact". Geology. 28 (12): 1119–1122. Bibcode:2000Geo....28.1119N. doi:10.1130/0091-7613(2000)28<1119:MFOTNA>2.0.CO;2.
- ^ Bryant, Edward (June 2014). Tsunami: The Underrated Hazard. Springer. p. 178. ISBN 978-3-319-06133-7. Archived from the original on 1 September 2019. Retrieved 30 August 2017.
- ^ Smit, Jan; Montanari, Alessandro; Swinburne, Nicola H.; Alvarez, Walter; Hildebrand, Alan R.; Margolis, Stanley V.; Claeys, Philippe; Lowrie, William; Asaro, Frank (1992). "Tektite-bearing, deep-water clastic unit at the Cretaceous-Tertiary boundary in northeastern Mexico". Geology. 20 (2): 99–103. Bibcode:1992Geo....20...99S. doi:10.1130/0091-7613(1992)020<0099:TBDWCU>2.3.CO;2. PMID 11537752.
- ^ Field guide to Cretaceous-tertiary boundary sections in northeastern Mexico (PDF). Lunar and Planetary Institute. 1994. Archived (PDF) from the original on 21 August 2019. Retrieved 25 June 2019.
- ^ Smit, Jan (1999). "The global stratigraphy of the Cretaceous-Tertiary boundary impact ejecta". Annual Review of Earth and Planetary Sciences. 27: 75–113. Bibcode:1999AREPS..27...75S. doi:10.1146/annurev.earth.27.1.75.
- ^ Kring, David A. (2007). "The Chicxulub impact event and its environmental consequences at the Cretaceous-Tertiary boundary". Palaeogeography, Palaeoclimatology, Palaeoecology. 255 (1–2): 4–21. Bibcode:2007PPP...255....4K. doi:10.1016/j.palaeo.2007.02.037.
- ^ "Chicxulub impact event". www.lpi.usra.edu. Archived from the original on 26 July 2019. Retrieved 25 June 2019.
- ^ Pope, K.O.; Baines, K.H.; Ocampo, A.C.; Ivanov, B. A. (1997). "Energy, volatile production, and climatic effects of the Chicxulub Cretaceous/Tertiary impact". Journal of Geophysical Research. 102 (E9): 21645–21664. Bibcode:1997JGR...10221645P. doi:10.1029/97JE01743. PMID 11541145.
- ^ Belcher, Claire M.; Hadden, Rory M.; Rein, Guillermo; Morgan, Joanna V.; Artemieva, Natalia; Goldin, Tamara (22 January 2015). "An experimental assessment of the ignition of forest fuels by the thermal pulse generated by the Cretaceous–Palaeogene impact at Chicxulub". Journal of the Geological Society. 172 (2): 175–185. doi:10.1144/jgs2014-082. hdl:10044/1/18936. ISSN 0016-7649.
- ^ Adair, Robert K. (1 June 2010). "Wildfires and animal extinctions at the Cretaceous/Tertiary boundary". American Journal of Physics. 78 (6): 567–573. doi:10.1119/1.3192770. ISSN 0002-9505. Retrieved 22 August 2024.
- ^ Morgan, Joanna V.; Bralower, Timothy J.; Brugger, Julia; Wünnemann, Kai (12 April 2022). "The Chicxulub impact and its environmental consequences". Nature Reviews Earth & Environment. 3 (5): 338–354. doi:10.1038/s43017-022-00283-y. ISSN 2662-138X. Retrieved 22 August 2024.
- ^ Kaiho, Kunio; Oshima, Naga (2017). "Site of asteroid impact changed the history of life on Earth: The low probability of mass extinction". Scientific Reports. 7 (1). Article number 14855. Bibcode:2017NatSR...714855K. doi:10.1038/s41598-017-14199-x. PMC 5680197. PMID 29123110.
- ^ Ohno, S.; Kadono, T.; Kurosawa, K.; et al. (2014). "Production of sulphate-rich vapour during the Chicxulub impact and implications for ocean acidification". Nature Geoscience. 7 (4): 279–282. Bibcode:2014NatGe...7..279O. doi:10.1038/ngeo2095.
- ^ Senel, Cem Berk; Kaskes, Pim; Temel, Orkun; Vellekoop, Johan; Goderis, Steven; DePalma, Robert; Prins, Maarten A.; Claeys, Philippe; Karatekin, Özgür (30 October 2023). "Chicxulub impact winter sustained by fine silicate dust". Nature Geoscience. 16 (11): 1033–1040. Bibcode:2023NatGe..16.1033S. doi:10.1038/s41561-023-01290-4. ISSN 1752-0894. S2CID 264805571.
- ^ Bardeen, Charles G.; Garcia, Rolando R.; Toon, Owen B.; Conley, Andrew J. (21 August 2017). "On transient climate change at the Cretaceous−Paleogene boundary due to atmospheric soot injections". Proceedings of the National Academy of Sciences of the United States. 114 (36): E7415–E7424. doi:10.1073/pnas.1708980114. ISSN 0027-8424. PMC 5594694. PMID 28827324.
- ^ Lyons, Shelby L.; Karp, Allison T.; Bralower, Timothy J.; Grice, Kliti; Schaefer, Bettina; Gulick, Sean P. S.; Morgan, Joanna V.; Freeman, Katherine H. (28 September 2020). "Organic matter from the Chicxulub crater exacerbated the K–Pg impact winter". Proceedings of the National Academy of Sciences of the United States of America. 117 (41): 25327–25334. Bibcode:2020PNAS..11725327L. doi:10.1073/pnas.2004596117. ISSN 0027-8424. PMC 7568312. PMID 32989138.
- ^ Kaiho, Kunio; Oshima, Naga; Adachi, Kouji; Adachi, Yukimasa; Mizukami, Takuya; Fujibayashi, Megumu; Saito, Ryosuke (14 July 2016). "Global climate change driven by soot at the K-Pg boundary as the cause of the mass extinction". Scientific Reports. 6 (1): 28427. Bibcode:2016NatSR...628427K. doi:10.1038/srep28427. ISSN 2045-2322. PMC 4944614. PMID 27414998.
- ^ Pierazzo, Elisabetta; Hahmann, Andrea N.; Sloan, Lisa C. (5 July 2004). "Chicxulub and Climate: Radiative Perturbations of Impact-Produced S-Bearing Gases". Astrobiology. 3 (1): 99–118. doi:10.1089/153110703321632453. ISSN 1531-1074. PMID 12804368.
- ^ Hand, Eric (17 November 2016). "Updated: Drilling of dinosaur-killing impact crater explains buried circular hills". Science. doi:10.1126/science.aaf5684.
- ^ "Chicxulub crater dinosaur extinction". The New York Times. New York, NY. 18 November 2016. Archived from the original on 9 November 2017. Retrieved 14 October 2017.
- ^ Sigurdsson, H.; D'Hondt, S.; Carey, S. (April 1992). "The impact of the Cretaceous/Tertiary bolide on evaporite terrane and generation of major sulfuric acid aerosol". Earth and Planetary Science Letters. 109 (3–4): 543–559. Bibcode:1992E&PSL.109..543S. doi:10.1016/0012-821X(92)90113-A.
- ^ Prinn, Ronald G.; Fegley, Bruce (May 1987). "Bolide impacts, acid rain, and biospheric traumas at the Cretaceous-Tertiary boundary". Earth and Planetary Science Letters. 83 (1–4): 1–15. Bibcode:1987E&PSL..83....1P. doi:10.1016/0012-821X(87)90046-X.
- ^ Pope, Kevin O.; Baines, Kevin H.; Ocampo, Adriana C.; Ivanov, Boris A. (December 1994). "Impact winter and the Cretaceous/Tertiary extinctions: Results of a Chicxulub asteroid impact model". Earth and Planetary Science Letters. 128 (3–4): 719–725. Bibcode:1994E&PSL.128..719P. doi:10.1016/0012-821X(94)90186-4. PMID 11539442.
- ^ Kawaragi, Ko; Sekine, Yasuhito; Kadono, Toshihiko; Sugita, Seiji; Ohno, Sohsuke; Ishibashi, Ko; Kurosawa, Kosuke; Matsui, Takafumi; Ikeda, Susumu (30 May 2009). "Direct measurements of chemical composition of shock-induced gases from calcite: an intense global warming after the Chicxulub impact due to the indirect greenhouse effect of carbon monoxide". Earth and Planetary Science Letters. 282 (1–4): 56–64. Bibcode:2009E&PSL.282...56K. doi:10.1016/j.epsl.2009.02.037.
- ^ Pierazzo, Elisabetta; Kring, David A.; Melosh, H. Jay (25 November 1998). "Hydrocode simulation of the Chicxulub impact event and the production of climatically active gases". Journal of Geophysical Research: Planets. 103 (E12): 28607–28625. Bibcode:1998JGR...10328607P. doi:10.1029/98JE02496. ISSN 0148-0227.
- ^ Brannen, Peter (2017). The Ends of the World: Volcanic Apocalypses, Lethal Oceans, and Our Quest to Understand Earth's Past Mass Extinctions. Harper Collins. p. 336. ISBN 978-0-06-236480-7.
- ^ Mullen, L. (13 October 2004). "Debating the Dinosaur Extinction". Astrobiology Magazine. Archived from the original on 25 June 2012. Retrieved 29 March 2012.
- ^ Mullen, L. (20 October 2004). "Multiple impacts". Astrobiology Magazine. Archived from the original on 6 April 2012. Retrieved 29 March 2012.
- ^ Mullen, L. (3 November 2004). "Shiva: Another K–T impact?". Astrobiology Magazine. Archived from the original on 11 December 2011. Retrieved 29 March 2012.
- ^ Chatterjee, Sankar (August 1997). "Multiple Impacts at the KT Boundary and the Death of the Dinosaurs". 30th International Geological Congress. Vol. 26. pp. 31–54. ISBN 978-90-6764-254-5.
- ^ Kaneoka, Ichiro (January 1980). "40Ar/39Ar dating on volcanic rocks of the Deccan Traps, India". Earth and Planetary Science Letters. 46 (2): 233–243. doi:10.1016/0012-821X(80)90009-6.
- ^ Duncan, R. A.; Pyle, D. G. (30 June 1988). "Rapid eruption of the Deccan flood basalts at the Cretaceous/Tertiary boundary". Nature. 333 (6176): 841–843. Bibcode:1988Natur.333..841D. doi:10.1038/333841a0. ISSN 0028-0836. S2CID 4351454.
- ^ Courtillot, V.; Féraud, G.; Maluski, H.; Vandamme, D.; Moreau, M. G.; Besse, J. (30 June 1988). "Deccan flood basalts and the Cretaceous/Tertiary boundary". Nature. 333 (6176): 843–846. Bibcode:1988Natur.333..843C. doi:10.1038/333843a0. ISSN 0028-0836. S2CID 4326163.
- ^ Courtillot, Vincent; Besse, Jean; Vandamme, Didier; Montigny, Raymond; Jaeger, Jean-Jacques; Cappetta, Henri (November 1986). "Deccan flood basalts at the Cretaceous/Tertiary boundary?". Earth and Planetary Science Letters. 80 (3–4): 361–374. Bibcode:1986E&PSL..80..361C. doi:10.1016/0012-821X(86)90118-4.
- ^ Courtillot, V. (December 1990). "Deccan volcanism at the Cretaceous-Tertiary boundary: past climatic crises as a key to the future?". Palaeogeography, Palaeoclimatology, Palaeoecology. 89 (3): 291–299. Bibcode:1990PPP....89..291C. doi:10.1016/0031-0182(90)90070-N.
- ^ Keller, G.; Adatte, T.; Gardin, S.; Bartolini, A.; Bajpai, S. (2008). "Main Deccan volcanism phase ends near the K–T boundary: Evidence from the Krishna-Godavari Basin, SE India". Earth and Planetary Science Letters. 268 (3–4): 293–311. Bibcode:2008E&PSL.268..293K. doi:10.1016/j.epsl.2008.01.015.
- ^ Callegaro, Sara; Baker, Don R.; Renne, Paul R.; Melluso, Leone; Geraki, Kalotina; Whitehouse, Martin J.; De Min, Angelo; Marzoli, Andrea (6 October 2023). "Recurring volcanic winters during the latest Cretaceous: Sulfur and fluorine budgets of Deccan Traps lavas". Science Advances. 9 (40): eadg8284. Bibcode:2023SciA....9G8284C. doi:10.1126/sciadv.adg8284. ISSN 2375-2548. PMC 10550224. PMID 37792933.
- ^ Nordt, Lee; Atchley, Stacy; Dworkin, Steve (December 2003). "Terrestrial Evidence for Two Greenhouse Events in the Latest Cretaceous". Geological Society of America Today. 13 (12): 4. doi:10.1130/1052-5173(2003)013<4:TEFTGE>2.0.CO;2. ISSN 1052-5173.
- ^ Berggren, W.A; Aubry, M.-P; van Fossen, M; Kent, D.V; Norris, R.D; Quillévéré, F (1 June 2000). "Integrated Paleocene calcareous plankton magnetobiochronology and stable isotope stratigraphy: DSDP Site 384 (NW Atlantic Ocean)". Palaeogeography, Palaeoclimatology, Palaeoecology. 159 (1–2): 1–51. Bibcode:2000PPP...159....1B. doi:10.1016/S0031-0182(00)00031-6.
- ^ Courtillot, Vincent (1990). "A volcanic eruption". Scientific American. 263 (4): 85–92. Bibcode:1990SciAm.263d..85C. doi:10.1038/scientificamerican1090-85. PMID 11536474.
- ^ Milligan, Joseph N.; Royer, Dana L.; Franks, Peter J.; Upchurch, Garland R.; McKee, Melissa L. (7 March 2019). "No Evidence for a Large Atmospheric CO2 Spike Across the Cretaceous-Paleogene Boundary". Geophysical Research Letters. 46 (6): 3462–3472. doi:10.1029/2018GL081215. ISSN 0094-8276.
- ^ Sial, A. N.; Lacerda, L. D.; Ferreira, V. P.; Frei, R.; Marquillas, R. A.; Barbosa, J. A.; Gaucher, C.; Windmöller, C. C.; Pereira, N. S. (1 October 2013). "Mercury as a proxy for volcanic activity during extreme environmental turnover: The Cretaceous–Paleogene transition". Palaeogeography, Palaeoclimatology, Palaeoecology. 387: 153–164. Bibcode:2013PPP...387..153S. doi:10.1016/j.palaeo.2013.07.019.
- ^ Keller, Gerta (July 2001). "The end-cretaceous mass extinction in the marine realm: Year 2000 assessment" (PDF). Planetary and Space Science. 49 (8): 817–830. Bibcode:2001P&SS...49..817K. doi:10.1016/S0032-0633(01)00032-0.
- ^ Adatte, Thierry; Keller, Gerta; Stinnesbeck, Wolfgang (28 February 2002). "Late Cretaceous to early Paleocene climate and sea-level fluctuations: the Tunisian record". Palaeogeography, Palaeoclimatology, Palaeoecology. 178 (3–4): 165–196. Bibcode:2002PPP...178..165A. doi:10.1016/S0031-0182(01)00395-9.
- ^ Keller, Gerta (October 1988). "Extinction, survivorship and evolution of planktic foraminifera across the Cretaceous/Tertiary boundary at El Kef, Tunisia". Marine Micropaleontology. 13 (3): 239–263. doi:10.1016/0377-8398(88)90005-9.
- ^ Zhang, Laiming; Wang, Chengshan; Wignall, Paul B.; Kluge, Tobias; Wan, Xiaoqiao; Wang, Qian; Gao, Yuan (1 March 2018). "Deccan volcanism caused coupled pCO2 and terrestrial temperature rises, and pre-impact extinctions in northern China". Geology. 46 (3): 271–274. Bibcode:2018Geo....46..271Z. doi:10.1130/G39992.1. ISSN 0091-7613.
- ^ Alvarez, W (1997). T. rex and the Crater of Doom. Princeton University Press. pp. 130–146. ISBN 978-0-691-01630-6.
- ^ Sprain, Courtney J.; Renne, Paul R.; Vanderkluysen, Loÿc; Pande, Kanchan; Self, Stephen; Mittal, Tushar (22 February 2019). "The eruptive tempo of Deccan volcanism in relation to the Cretaceous-Paleogene boundary". Science. 363 (6429): 866–870. Bibcode:2019Sci...363..866S. doi:10.1126/science.aav1446. ISSN 0036-8075. PMID 30792301.
- ^ Cripps, J.A.; Widdowson, M.; Spicer, R.A.; Jolley, D.W. (1 February 2005). "Coastal ecosystem responses to late stage Deccan Trap volcanism: the post K–T boundary (Danian) palynofacies of Mumbai (Bombay), west India". Palaeogeography, Palaeoclimatology, Palaeoecology. 216 (3–4): 303–332. Bibcode:2005PPP...216..303C. doi:10.1016/j.palaeo.2004.11.007.
- ^ Dzombak, R.M.; Sheldon, N.D.; Mohabey, D.M.; Samant, B. (September 2020). "Stable climate in India during Deccan volcanism suggests limited influence on K–Pg extinction". Gondwana Research. 85: 19–31. Bibcode:2020GondR..85...19D. doi:10.1016/j.gr.2020.04.007.
- ^ Renne, P. R.; et al. (2015). "State shift in Deccan volcanism at the Cretaceous-Paleogene boundary, possibly induced by impact". Science. 350 (6256): 76–78. Bibcode:2015Sci...350...76R. doi:10.1126/science.aac7549. PMID 26430116.
- ^ Richards, M. A.; et al. (2015). "Triggering of the largest Deccan eruptions by the Chicxulub impact" (PDF). Geological Society of America Bulletin. 127 (11–12): 1507–1520. Bibcode:2015GSAB..127.1507R. doi:10.1130/B31167.1. S2CID 3463018.
- ^ Li, Liangquan; Keller, Gerta (1998). "Abrupt deep-sea warming at the end of the Cretaceous". Geology. 26 (11): 995–998. Bibcode:1998Geo....26..995L. doi:10.1130/0091-7613(1998)026<0995:ADSWAT>2.3.CO;2. S2CID 115136793.
- ^ Keller, Gerta (June 1989). "Extended Cretaceous/Tertiary boundary extinctions and delayed population change in planktonic foraminifera from Brazos River, Texas". Paleoceanography and Paleoclimatology. 4 (3): 287–332. doi:10.1029/PA004i003p00287. ISSN 0883-8305.
- ^ Keller, Gerta; Barrera, Enriqueta (1990). "The Cretaceous/Tertiary boundary impact hypothesis and the paleontological record". In Sharpton, Virgil L.; Ward, Peter D. (eds.). Global Catastrophes in Earth History; An Interdisciplinary Conference on Impacts, Volcanism, and Mass Mortality. Geological Society of America. doi:10.1130/SPE247-p563. ISBN 9780813722474.
- ^ Jiang, M. J.; Gartner, S. (1986). "Calcareous Nannofossil Succession across the Cretaceous/Tertiary Boundary in East-Central Texas". Micropaleontology. 32 (3): 232. doi:10.2307/1485619. JSTOR 1485619.
- ^ Petersen, Sierra V.; Dutton, Andrea; Lohmann, Kyger C. (2016). "End-Cretaceous extinction in Antarctica linked to both Deccan volcanism and meteorite impact via climate change". Nature Communications. 7: 12079. Bibcode:2016NatCo...712079P. doi:10.1038/ncomms12079. PMC 4935969. PMID 27377632.
Further reading
- Cowen, R. (2000). "The K–T extinction". University of California Museum of Paleontology. Retrieved 2 August 2007.
- DePalma, Robert A.; et al. (1 April 2019). "A seismically induced onshore surge deposit at the KPg boundary, North Dakota". PNAS. 116 (17): 8190–8199. Bibcode:2019PNAS..116.8190D. doi:10.1073/pnas.1817407116. PMC 6486721. PMID 30936306.
- Fortey, Richard (2005). Earth: An Intimate History. New York: Vintage Books. ISBN 978-0-375-70620-2. OCLC 54537112.
- Papers and presentations resulting from the 2016 Chicxulub drilling project—The Geological Society of America, GSA Annual Meeting in Seattle, Washington, USA – 2017, Session No. 192
- Kring, D.A. (2005). "Chicxulub impact event: Understanding the K–T boundary". NASA Space Imagery Center. Archived from the original on 29 June 2007. Retrieved 2 August 2007.
- Preston, Douglas (8 April 2019). "The Day the Dinosaurs Died". The New Yorker. pp. 52–65.
Robert A. de Palm has found strong evidence that the dinosaurs—and nearly all other life on Earth—were indeed wiped out 66 million years ago by the Chicxulub asteroid
External links
- What killed the dinosaurs?—University of California Museum of Paleontology (1995)
- The Great Chicxulub Debate 2004—Geological Society of London