Yeasts
- Saccharomycotina (true yeasts)
- Taphrinomycotina p. p.
- Schizosaccharomycetes (fission yeasts)
Basidiomycota p. p.
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to constitute 1% of all described fungal species.
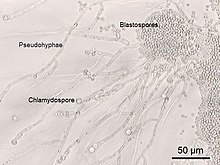
Some yeast species have the ability to develop multicellular characteristics by forming strings of connected budding cells known as pseudohyphae or false hyphae, or quickly evolve into a multicellular cluster with specialised cell organelles function. Yeast sizes vary greatly, depending on species and environment, typically measuring 3–4 μm in diameter, although some yeasts can grow to 40 μm in size. Most yeasts reproduce asexually by mitosis, and many do so by the asymmetric division process known as budding. With their single-celled growth habit, yeasts can be contrasted with molds, which grow hyphae. Fungal species that can take both forms (depending on temperature or other conditions) are called dimorphic fungi.
The yeast species Saccharomyces cerevisiae converts carbohydrates to carbon dioxide and alcohols through the process of fermentation. The products of this reaction have been used in baking and the production of alcoholic beverages for thousands of years. S. cerevisiae is also an important model organism in modern cell biology research, and is one of the most thoroughly studied eukaryotic microorganisms. Researchers have cultured it in order to understand the biology of the eukaryotic cell and ultimately human biology in great detail. Other species of yeasts, such as Candida albicans, are opportunistic pathogens and can cause infections in humans. Yeasts have recently been used to generate electricity in microbial fuel cells and to produce ethanol for the biofuel industry.
Yeasts do not form a single taxonomic or phylogenetic grouping. The term "yeast" is often taken as a synonym for Saccharomyces cerevisiae, but the phylogenetic diversity of yeasts is shown by their placement in two separate phyla: the Ascomycota and the Basidiomycota. The budding yeasts or "true yeasts" are classified in the order Saccharomycetales, within the phylum Ascomycota.
History
The word "yeast" comes from Old English gist, gyst, and from the Indo-European root yes-, meaning "boil", "foam", or "bubble". Yeast microbes are probably one of the earliest domesticated organisms. Archaeologists digging in Egyptian ruins found early grinding stones and baking chambers for yeast-raised bread, as well as drawings of 4,000-year-old bakeries and breweries. Vessels studied from several archaeological sites in Israel (dating to around 5,000, 3,000 and 2,500 years ago), which were believed to have contained alcoholic beverages (beer and mead), were found to contain yeast colonies that had survived over the millennia, providing the first direct biological evidence of yeast use in early cultures. In 1680, Dutch naturalist Anton van Leeuwenhoek first microscopically observed yeast, but at the time did not consider them to be living organisms, but rather globular structures as researchers were doubtful whether yeasts were algae or fungi. Theodor Schwann recognized them as fungi in 1837.
In 1857, French microbiologist Louis Pasteur showed that by bubbling oxygen into the yeast broth, cell growth could be increased, but fermentation was inhibited – an observation later called the "Pasteur effect". In the paper "Mémoire sur la fermentation alcoolique," Pasteur proved that alcoholic fermentation was conducted by living yeasts and not by a chemical catalyst.
By the late 18th century two yeast strains used in brewing had been identified: Saccharomyces cerevisiae (top-fermenting yeast) and S. pastorianus (bottom-fermenting yeast). S. cerevisiae has been sold commercially by the Dutch for bread-making since 1780; while, around 1800, the Germans started producing S. cerevisiae in the form of cream. In 1825, a method was developed to remove the liquid so the yeast could be prepared as solid blocks. The industrial production of yeast blocks was enhanced by the introduction of the filter press in 1867. In 1872, Baron Max de Springer developed a manufacturing process to create granulated yeast from beetroot molasses, a technique that was used until the first World War. In the United States, naturally occurring airborne yeasts were used almost exclusively until commercial yeast was marketed at the Centennial Exposition in 1876 in Philadelphia, where Charles L. Fleischmann exhibited the product and a process to use it, as well as serving the resultant baked bread.
The mechanical refrigerator (first patented in the 1850s in Europe) liberated brewers and winemakers from seasonal constraints for the first time and allowed them to exit cellars and other earthen environments. For John Molson, who made his livelihood in Montreal prior to the development of the fridge, the brewing season lasted from September through to May. The same seasonal restrictions formerly governed the distiller's art.
Nutrition and growth
Yeasts are chemoorganotrophs, as they use organic compounds as a source of energy and do not require sunlight to grow. Carbon is obtained mostly from hexose sugars, such as glucose and fructose, or disaccharides such as sucrose and maltose. Some species can metabolize pentose sugars such as ribose, alcohols, and organic acids. Yeast species either require oxygen for aerobic cellular respiration (obligate aerobes) or are anaerobic, but also have aerobic methods of energy production (facultative anaerobes). Unlike bacteria, no known yeast species grow only anaerobically (obligate anaerobes). Most yeasts grow best in a neutral or slightly acidic pH environment.
Yeasts vary in regard to the temperature range in which they grow best. For example, Leucosporidium frigidum grows at −2 to 20 °C (28 to 68 °F), Saccharomyces telluris at 5 to 35 °C (41 to 95 °F), and Candida slooffi at 28 to 45 °C (82 to 113 °F). The cells can survive freezing under certain conditions, with viability decreasing over time.
In general, yeasts are grown in the laboratory on solid growth media or in liquid broths. Common media used for the cultivation of yeasts include potato dextrose agar or potato dextrose broth, Wallerstein Laboratories nutrient agar, yeast peptone dextrose agar, and yeast mould agar or broth. Home brewers who cultivate yeast frequently use dried malt extract and agar as a solid growth medium. The fungicide cycloheximide is sometimes added to yeast growth media to inhibit the growth of Saccharomyces yeasts and select for wild/indigenous yeast species. This will change the yeast process.
The appearance of a white, thready yeast, commonly known as kahm yeast, is often a byproduct of the lactofermentation (or pickling) of certain vegetables. It is usually the result of exposure to air. Although harmless, it can give pickled vegetables a bad flavor and must be removed regularly during fermentation.
Ecology
Yeasts are very common in the environment, and are often isolated from sugar-rich materials. Examples include naturally occurring yeasts on the skins of fruits and berries (such as grapes, apples, or peaches), and exudates from plants (such as plant saps or cacti). Some yeasts are found in association with soil and insects. Yeasts from the soil and from the skins of fruits and berries have been shown to dominate fungal succession during fruit decay. The ecological function and biodiversity of yeasts are relatively unknown compared to those of other microorganisms. Yeasts, including Candida albicans, Rhodotorula rubra, Torulopsis and Trichosporon cutaneum, have been found living in between people's toes as part of their skin flora. Yeasts are also present in the gut flora of mammals and some insects and even deep-sea environments host an array of yeasts.
An Indian study of seven bee species and nine plant species found 45 species from 16 genera colonize the nectaries of flowers and honey stomachs of bees. Most were members of the genus Candida; the most common species in honey stomachs was Dekkera intermedia and in flower nectaries, Candida blankii. Yeast colonising nectaries of the stinking hellebore have been found to raise the temperature of the flower, which may aid in attracting pollinators by increasing the evaporation of volatile organic compounds. A black yeast has been recorded as a partner in a complex relationship between ants, their mutualistic fungus, a fungal parasite of the fungus and a bacterium that kills the parasite. The yeast has a negative effect on the bacteria that normally produce antibiotics to kill the parasite, so may affect the ants' health by allowing the parasite to spread.
Certain strains of some species of yeasts produce proteins called yeast killer toxins that allow them to eliminate competing strains. (See main article on killer yeast.) This can cause problems for winemaking but could potentially also be used to advantage by using killer toxin-producing strains to make the wine. Yeast killer toxins may also have medical applications in treating yeast infections (see "Pathogenic yeasts" section below).
Marine yeasts, defined as the yeasts that are isolated from marine environments, are able to grow better on a medium prepared using seawater rather than freshwater. The first marine yeasts were isolated by Bernhard Fischer in 1894 from the Atlantic Ocean, and those were identified as Torula sp. and Mycoderma sp. Following this discovery, various other marine yeasts have been isolated from around the world from different sources, including seawater, seaweeds, marine fish and mammals. Among these isolates, some marine yeasts originated from terrestrial habitats (grouped as facultative marine yeast), which were brought to and survived in marine environments. The other marine yeasts were grouped as obligate or indigenous marine yeasts, which are confined to marine habitats. However, no sufficient evidence has been found to explain the indispensability of seawater for obligate marine yeasts. It has been reported that marine yeasts are able to produce many bioactive substances, such as amino acids, glucans, glutathione, toxins, enzymes, phytase, and vitamins with potential applications in the food, pharmaceutical, cosmetic, and chemical industries as well as for marine culture and environmental protection. Marine yeast was successfully used to produce bioethanol using seawater-based media which will potentially reduce the water footprint of bioethanol.
Reproduction

- Budding
- Conjugation
- Spore
Yeasts, like all fungi, may have asexual and sexual reproductive cycles. The most common mode of vegetative growth in yeast is asexual reproduction by budding, where a small bud (also known as a bleb or daughter cell) is formed on the parent cell. The nucleus of the parent cell splits into a daughter nucleus and migrates into the daughter cell. The bud then continues to grow until it separates from the parent cell, forming a new cell. The daughter cell produced during the budding process is generally smaller than the mother cell. Some yeasts, including Schizosaccharomyces pombe, reproduce by fission instead of budding, and thereby creating two identically sized daughter cells.
In general, under high-stress conditions such as nutrient starvation, haploid cells will die; under the same conditions, however, diploid cells can undergo sporulation, entering sexual reproduction (meiosis) and producing a variety of haploid spores, which can go on to mate (conjugate), reforming the diploid.
The haploid fission yeast Schizosaccharomyces pombe is a facultative sexual microorganism that can undergo mating when nutrients are limited. Exposure of S. pombe to hydrogen peroxide, an agent that causes oxidative stress leading to oxidative DNA damage, strongly induces mating and the formation of meiotic spores. The budding yeast Saccharomyces cerevisiae reproduces by mitosis as diploid cells when nutrients are abundant, but when starved, this yeast undergoes meiosis to form haploid spores. Haploid cells may then reproduce asexually by mitosis. Katz Ezov et al. presented evidence that in natural S. cerevisiae populations clonal reproduction and selfing (in the form of intratetrad mating) predominate. In nature, the mating of haploid cells to form diploid cells is most often between members of the same clonal population and out-crossing is uncommon. Analysis of the ancestry of natural S. cerevisiae strains led to the conclusion that out-crossing occurs only about once every 50,000 cell divisions. These observations suggest that the possible long-term benefits of outcrossing (e.g. generation of diversity) are likely to be insufficient for generally maintaining sex from one generation to the next. Rather, a short-term benefit, such as recombinational repair during meiosis, may be the key to the maintenance of sex in S. cerevisiae.
Some pucciniomycete yeasts, in particular species of Sporidiobolus and Sporobolomyces, produce aerially dispersed, asexual ballistoconidia.
Uses
The useful physiological properties of yeast have led to their use in the field of biotechnology. Fermentation of sugars by yeast is the oldest and largest application of this technology. Many types of yeasts are used for making many foods: baker's yeast in bread production, brewer's yeast in beer fermentation, and yeast in wine fermentation and for xylitol production. So-called red rice yeast is actually a mold, Monascus purpureus. Yeasts include some of the most widely used model organisms for genetics and cell biology.
Alcoholic beverages
Alcoholic beverages are defined as beverages that contain ethanol (C2H5OH). This ethanol is almost always produced by fermentation – the metabolism of carbohydrates by certain species of yeasts under anaerobic or low-oxygen conditions. Beverages such as mead, wine, beer, or distilled spirits all use yeast at some stage of their production. A distilled beverage is a beverage containing ethanol that has been purified by distillation. Carbohydrate-containing plant material is fermented by yeast, producing a dilute solution of ethanol in the process. Spirits such as whiskey and rum are prepared by distilling these dilute solutions of ethanol. Components other than ethanol are collected in the condensate, including water, esters, and other alcohols, which (in addition to that provided by the oak in which it may be aged) account for the flavour of the beverage.
Beer


Brewing yeasts may be classed as "top-cropping" (or "top-fermenting") and "bottom-cropping" (or "bottom-fermenting"). Top-cropping yeasts are so called because they form a foam at the top of the wort during fermentation. An example of a top-cropping yeast is Saccharomyces cerevisiae, sometimes called an "ale yeast". Bottom-cropping yeasts are typically used to produce lager-type beers, though they can also produce ale-type beers. These yeasts ferment well at low temperatures. An example of bottom-cropping yeast is Saccharomyces pastorianus, formerly known as S. carlsbergensis.
Decades ago, taxonomists reclassified S. carlsbergensis (uvarum) as a member of S. cerevisiae, noting that the only distinct difference between the two is metabolic. Lager strains of S. cerevisiae secrete an enzyme called melibiase, allowing them to hydrolyse melibiose, a disaccharide, into more fermentable monosaccharides. Top- and bottom-cropping and cold- and warm-fermenting distinctions are largely generalizations used by laypersons to communicate to the general public.
The most common top-cropping brewer's yeast, S. cerevisiae, is the same species as the common baking yeast. Brewer's yeast is also very rich in essential minerals and the B vitamins (except B12), a feature exploited in food products made from leftover (by-product) yeast from brewing. However, baking and brewing yeasts typically belong to different strains, cultivated to favour different characteristics: baking yeast strains are more aggressive, to carbonate dough in the shortest amount of time possible; brewing yeast strains act more slowly but tend to produce fewer off-flavours and tolerate higher alcohol concentrations (with some strains, up to 22%).
Dekkera/Brettanomyces is a genus of yeast known for its important role in the production of 'lambic' and specialty sour ales, along with the secondary conditioning of a particular Belgian Trappist beer. The taxonomy of the genus Brettanomyces has been debated since its early discovery and has seen many reclassifications over the years. Early classification was based on a few species that reproduced asexually (anamorph form) through multipolar budding. Shortly after, the formation of ascospores was observed and the genus Dekkera, which reproduces sexually (teleomorph form), was introduced as part of the taxonomy. The current taxonomy includes five species within the genera of Dekkera/Brettanomyces. Those are the anamorphs Brettanomyces bruxellensis, Brettanomyces anomalus, Brettanomyces custersianus, Brettanomyces naardenensis, and Brettanomyces nanus, with teleomorphs existing for the first two species, Dekkera bruxellensis and Dekkera anomala. The distinction between Dekkera and Brettanomyces is arguable, with Oelofse et al. (2008) citing Loureiro and Malfeito-Ferreira from 2006 when they affirmed that current molecular DNA detection techniques have uncovered no variance between the anamorph and teleomorph states. Over the past decade, Brettanomyces spp. have seen an increasing use in the craft-brewing sector of the industry, with a handful of breweries having produced beers that were primarily fermented with pure cultures of Brettanomyces spp. This has occurred out of experimentation, as very little information exists regarding pure culture fermentative capabilities and the aromatic compounds produced by various strains. Dekkera/Brettanomyces spp. have been the subjects of numerous studies conducted over the past century, although a majority of the recent research has focused on enhancing the knowledge of the wine industry. Recent research on eight Brettanomyces strains available in the brewing industry focused on strain-specific fermentations and identified the major compounds produced during pure culture anaerobic fermentation in wort.
Wine

Yeast is used in winemaking, where it converts the sugars present (glucose and fructose) in grape juice (must) into ethanol. Yeast is normally already present on grape skins. Fermentation can be done with this endogenous "wild yeast", but this procedure gives unpredictable results, which depend upon the exact types of yeast species present. For this reason, a pure yeast culture is usually added to the must; this yeast quickly dominates the fermentation. The wild yeasts are repressed, which ensures a reliable and predictable fermentation.
Most added wine yeasts are strains of S. cerevisiae, though not all strains of the species are suitable. Different S. cerevisiae yeast strains have differing physiological and fermentative properties, therefore the actual strain of yeast selected can have a direct impact on the finished wine. Significant research has been undertaken into the development of novel wine yeast strains that produce atypical flavour profiles or increased complexity in wines.
The growth of some yeasts, such as Zygosaccharomyces and Brettanomyces, in wine can result in wine faults and subsequent spoilage. Brettanomyces produces an array of metabolites when growing in wine, some of which are volatile phenolic compounds. Together, these compounds are often referred to as "Brettanomyces character", and are often described as "antiseptic" or "barnyard" type aromas. Brettanomyces is a significant contributor to wine faults within the wine industry.
Researchers from the University of British Columbia, Canada, have found a new strain of yeast that has reduced amines. The amines in red wine and Chardonnay produce off-flavors and cause headaches and hypertension in some people. About 30% of people are sensitive to biogenic amines, such as histamines.
Baking
This section needs additional citations for verification. (April 2013) |
Yeast, most commonly S. cerevisiae, is used in baking as a leavening agent, converting the fermentable sugars present in dough into carbon dioxide. This causes the dough to expand or rise as gas forms pockets or bubbles. When the dough is baked, the yeast dies and the air pockets "set", giving the baked product a soft and spongy texture. The use of potatoes, water from potato boiling, eggs, or sugar in a bread dough accelerates the growth of yeast. Most yeasts used in baking are of the same species common in alcoholic fermentation. In addition, Saccharomyces exiguus (also known as S. minor), a wild yeast found on plants, fruits, and grains, is occasionally used for baking. In breadmaking, the yeast initially respires aerobically, producing carbon dioxide and water. When the oxygen is depleted, fermentation begins, producing ethanol as a waste product; however, this evaporates during baking.

It is not known when yeast was first used to bake bread. The first records that show this use came from Ancient Egypt. Researchers speculate a mixture of flour meal and water was left longer than usual on a warm day and the yeasts that occur in natural contaminants of the flour caused it to ferment before baking. The resulting bread would have been lighter and tastier than the normal flat, hard cake.

Today, there are several retailers of baker's yeast; one of the earlier developments in North America is Fleischmann's Yeast, in 1868. During World War II, Fleischmann's developed a granulated active dry yeast which did not require refrigeration, had a longer shelf life than fresh yeast, and rose twice as fast. Baker's yeast is also sold as a fresh yeast compressed into a square "cake". This form perishes quickly, so must be used soon after production. A weak solution of water and sugar can be used to determine whether yeast is expired. In the solution, active yeast will foam and bubble as it ferments the sugar into ethanol and carbon dioxide. Some recipes refer to this as proofing the yeast, as it "proves" (tests) the viability of the yeast before the other ingredients are added. When a sourdough starter is used, flour and water are added instead of sugar; this is referred to as proofing the sponge.
When yeast is used for making bread, it is mixed with flour, salt, and warm water or milk. The dough is kneaded until it is smooth, and then left to rise, sometimes until it has doubled in size. The dough is then shaped into loaves. Some bread doughs are knocked back after one rising and left to rise again (this is called dough proofing) and then baked. A longer rising time gives a better flavor, but the yeast can fail to raise the bread in the final stages if it is left for too long initially.
Bioremediation
Some yeasts can find potential application in the field of bioremediation. One such yeast, Yarrowia lipolytica, is known to degrade palm oil mill effluent, TNT (an explosive material), and other hydrocarbons, such as alkanes, fatty acids, fats and oils. It can also tolerate high concentrations of salt and heavy metals, and is being investigated for its potential as a heavy metal biosorbent. Saccharomyces cerevisiae has potential to bioremediate toxic pollutants like arsenic from industrial effluent. Bronze statues are known to be degraded by certain species of yeast. Different yeasts from Brazilian gold mines bioaccumulate free and complexed silver ions.
Industrial ethanol production
The ability of yeast to convert sugar into ethanol has been harnessed by the biotechnology industry to produce ethanol fuel. The process starts by milling a feedstock, such as sugar cane, field corn, or other cereal grains, and then adding dilute sulfuric acid, or fungal alpha amylase enzymes, to break down the starches into complex sugars. A glucoamylase is then added to break the complex sugars down into simple sugars. After this, yeasts are added to convert the simple sugars to ethanol, which is then distilled off to obtain ethanol up to 96% in purity.
Saccharomyces yeasts have been genetically engineered to ferment xylose, one of the major fermentable sugars present in cellulosic biomasses, such as agriculture residues, paper wastes, and wood chips. Such a development means ethanol can be efficiently produced from more inexpensive feedstocks, making cellulosic ethanol fuel a more competitively priced alternative to gasoline fuels.