Slime Molds
The slime mold life cycle includes a free-living single-celled stage and the formation of spores. Spores are often produced in macroscopic multicellular or multinucleate fruiting bodies that may be formed through aggregation or fusion; aggregation is driven by chemical signals called acrasins. Slime molds contribute to the decomposition of dead vegetation; some are parasitic.
Most slime molds are terrestrial and free-living, typically in damp shady habitats such as in or on the surface of rotting wood. Some myxogastrians and protostelians are aquatic or semi-aquatic. The phytomyxea are parasitic, living inside their plant hosts. Geographically, slime molds are cosmopolitan in distribution. A small number of species occur in regions as dry as the Atacama Desert and as cold as the Arctic; they are abundant in the tropics, especially in rainforests.
Slime molds have a variety of behaviors otherwise seen in animals with brains. Species such as Physarum polycephalum have been used to simulate traffic networks. Some species have traditionally been eaten in countries such as Ecuador.
Evolution
Taxonomic history

The first account of slime molds was Thomas Panckow's 1654 discussion of Lycogala epidendrum. He called it Fungus cito crescentes, "a fast-growing fungus".
German mycologist Heinrich Anton de Bary, in 1860 and 1887, classified the Myxomycetes (plasmodial slime molds) and Acrasieae (cellular slime molds) as Mycetozoa, a new class. He also introduced a "Doubtful Mycetozoa" section for Plasmodiophora (now in Phytomyxea) and Labyrinthula, emphasizing their distinction from plants and fungi. In 1880, the French botanist Philippe van Tieghem analyzed the two groups further.
In 1868, the German biologist Ernst Haeckel placed the Mycetozoa in a kingdom he named Protista. In 1885, the British zoologist Ray Lankester grouped the Mycetozoa alongside the Proteomyxa as part of the Gymnomyxa in the phylum Protozoa. Arthur and Gulielma Lister published monographs of the group in 1894, 1911, and 1925.
In 1932 and 1960, the American mycologist George Willard Martin argued that the slime molds evolved from fungi. In 1956, the American biologist Herbert Copeland placed the Mycetozoa (the myxomycetes and plasmodiophorids) and the Sarkodina (the labyrinthulids and the cellular slime molds) in a phylum called Protoplasta, which he placed alongside the fungi and the algae in a new kingdom, Protoctista.
In 1969, the taxonomist R. H. Whittaker observed that slime molds were highly conspicuous and distinct within the Fungi, the group to which they were then classified. He concurred with Lindsay S. Olive's proposal to reclassify the Gymnomycota, which includes slime molds, as part of the Protista. Whittaker placed three phyla, namely the Myxomycota, Acrasiomycota, and Labyrinthulomycota in a subkingdom Gymnomycota within the Fungi. The same year, Martin and Alexopoulos published their influential textbook The Myxomycetes.
In 1975, Olive distinguished the dictyostelids and the acrasids as separate groups. In 1992, David J. Patterson and M. L. Sogin proposed that the dictyostelids diverged before plants, animals, and fungi.
Phylogeny
Slime molds have little or no fossil history, as might be expected given that they are small and soft-bodied. The grouping is polyphyletic, consisting of multiple clades (emphasised in the phylogenetic tree) widely scattered across the Eukaryotes. Paraphyletic groups are shown in quotation marks:
Diversity
Various estimates of the number of species of slime molds agree that there are around 1000 species, most being Myxogastria. Collection of environmental DNA gives a higher estimate, from 1200 to 1500 species. These are diverse both taxonomically and in appearance, the largest and most familiar species being among the Myxogastria. The growth forms most commonly noticed are the sporangia, the spore-forming bodies, which are often roughly spherical; these may be directly on the surface, such as on rotting wood, or may be on a thin stalk which elevates the spores for release above the surface. Other species have the spores in a large mass, which may be visited by insects for food; they disperse spores when they leave.
Macroscopic, plasmodial slime molds: Myxogastria
The Myxogastria or plasmodial slime molds are the only macroscopic scale slime molds; they gave the group its informal name, since for part of their life cycle they are slimy to the touch. A myxogastrian consists of a large cell with thousands of nuclei within a single membrane without walls, forming a syncytium. Most are smaller than a few centimeters, but some species may reach sizes up to several square meters, and in the case of Brefeldia maxima, a mass of up to 20 kilograms (44 lb).
-
Stemonitis shows stalked sporangia for airborne spore dispersal.
-
Fuligo septica cells aggregate to form a soft mass.
-
Enteridium lycoperdon sporangium. Spores can disperse in air or water, or by slime mold flies.
-
Metatrichia vesparium has small round sporangia that have spiral elaters to eject their lids and disperse their spores.
-
Mucilago crustacea aggregating from a streaming plasmodium (network of filaments) to a sporangium (large mass)
Cellular slime molds: Dictyosteliida
The Dictyosteliida or cellular slime molds do not form huge coenocytes like the Myxogastria; their amoebae remain individual for most of their lives as individual unicellular protists, feeding on microorganisms. When food is depleted and they are ready to form sporangia, they form swarms. The amoebae join up into a tiny multicellular slug which crawls to an open lit place and grows into a fruiting body, a sorocarp. Some of the amoebae become spores to begin the next generation, but others sacrifice themselves to become a dead stalk, lifting the spores up into the air.
-
Dictyostelium discoideum is a microscopic organism. The cells can aggregate to form a grex or slug, and then to a sorocarp or fruiting body (shown) on a delicate stalk.
Protosteliida
The Protosteliida, a polyphyletic group, have characters intermediate between the previous two groups, but they are much smaller, the fruiting bodies only forming one to a few spores.
-
Ceratiomyxa is microscopic; each stalk is topped by only one or a very few spores.
Copromyxa
The lobosans, a paraphyletic group of amoebae, include the Copromyxa slime molds.
Non-amoebozoan slime molds
Among the non-amoebozoan slime molds are the Acrasids, which have sluglike amoebae. In locomotion, the amoebae's pseudopodia are eruptive, meaning that hemispherical bulges appear at the front. The Phytomyxea are obligate parasites, with hosts among the plants, diatoms, oomycetes, and brown algae. They cause plant diseases like cabbage club root and powdery scab. The Labyrinthulomycetes are marine slime nets, forming labyrinthine networks of tubes in which amoeba without pseudopods can travel. The Fonticulida are cellular slime molds that form a fruiting body in a "volcano" shape.
-
The Labyrinthulomycete Aplanochytrium is a marine protist.
Distribution, habitats, and ecology

Slime molds, with their small size and moist surface, live mostly in damp habitats including shaded forests, rotting wood, fallen or living leaves, and on bryophytes. Most Myxogastria are terrestrial, though some, like Didymium aquatilis are aquatic, and D. nigripes is semi-aquatic. Myxogastria are not limited to wet regions; 34 species are known from Saudi Arabia, living on bark, in plant litter, and rotting wood, even in deserts. They occur, too, in Arizona's Sonoran Desert (46 species), and in Chile's exceptionally dry Atacama Desert (24 species). In contrast, the semi-dry Tehuacán-Cuicatlán Biosphere Reserve has 105 species, and Russia and Kazakhstan's Volga river basin has 158 species. In tropical rainforests of Latin America, species such as of Arcyria and Didymium are commonly epiphyllous, growing on the leaves of liverworts.
The dictyostelids are mostly terrestrial. On Changbai Mountain in China, six species of dictyostelids were found in forest soils at elevations up to 2,038 m (6,686 ft), the highest recorded species there being Dictyostelium mucoroides. The protostelids live mainly on dead plant matter, where they consume the spores of bacteria, yeasts, and fungi. They include some aquatic species, which live on dead plant parts submerged in ponds. Cellular slime molds are most numerous in the tropics, decreasing with latitude, but are cosmopolitan in distribution, occurring in soil even in the Arctic and the Antarctic. In the Alaskan tundra, the only slime molds are the dictyostelids D. mucoroides and D. sphaerocephalum.
The species of Copromyxa are coprophilous, feeding on dung.
Some myxogastrians have their spores dispersed by animals. The slime mold fly Epicypta testata lay its eggs within the spore mass of Enteridium lycoperdon, which the larvae feed on. These pupate, and the hatching adults carry and disperse spores that have stuck to them. While various insects consume slime molds, Sphindidae slime mold beetles, both larvae and adults, exclusively feed on them.
Life cycle
Plasmodial slime molds

Plasmodial slime molds begin life as amoeba-like cells. These unicellular amoebae are commonly haploid and feed on small prey such as bacteria, yeast cells, and fungal spores by phagocytosis, engulfing them with its cell membrane. These amoebae can mate if they encounter the correct mating type and form zygotes that then grow into plasmodia. These contain many nuclei without cell membranes between them, and can grow to meters in size. The species Fuligo septica is often seen as a slimy yellow network in and on rotting logs. The amoebae and the plasmodia engulf microorganisms. The plasmodium grows into an interconnected network of protoplasmic strands. Within each protoplasmic strand, the cytoplasmic contents rapidly stream, periodically reversing direction. The streaming protoplasm within a plasmodial strand can reach speeds of up to 1.35 mm per second in Physarum polycephalum, the fastest for any microorganism.
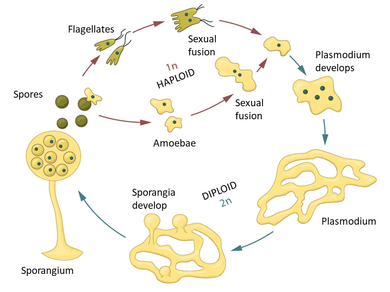
Slime molds are isogamous, which means that their gametes (reproductive cells) are all the same size, unlike the eggs and sperms of animals. Physarum polycephalum has three genes involved in reproduction: matA and matB, with thirteen variants each, and matC with three variants. Each reproductively mature slime mold is diploid, meaning that it contains two copies of each of the three reproductive genes. When P. polycephalum is ready to make its reproductive cells, it grows a bulbous extension of its body to contain them. Each cell has a random combination of the genes that the slime mold contains within its genome. Therefore, it can create cells of up to eight different gene types. Released cells then independently seek another compatible cell for fusion. Other individuals of P. polycephalum may contain different combinations of the matA, matB, and matC genes, allowing over 500 possible variations. It is advantageous for organisms with this type of reproductive cell to have many mating types because the likelihood of the cells finding a partner is greatly increased, and the risk of inbreeding is drastically reduced.
Cellular slime molds
The cellular slime molds are a group of approximately 150 described species. They occur primarily in the humus layer of forest soils and feed on bacteria but also are found in animal dung and agricultural fields. They exist as single-celled organisms while food is plentiful. When food is in short supply, many of the single-celled amoebae congregate and start moving as a single body, called a 'slug'. The ability of the single celled organisms to aggregate into multicellular forms are why they are also called the social amoebae. In this state they are sensitive to airborne chemicals and can detect food sources. They readily change the shape and function of parts, and may form stalks that produce fruiting bodies, releasing countless spores, light enough to be carried on the wind or on passing animals. The cellular slime mold Dictyostelium discoideum has many different mating types. When this organism has entered the stage of reproduction, it releases a chemical attractant. When it comes time for the cells to fuse, Dictyostelium discoideum has mating types of its own that dictate which cells are compatible with each other. There are at least eleven mating types; macrocysts form after cell contact between compatible mating types.
Chemical signals

The chemicals that aggregate cellular slime molds are small molecules called acrasins; motion towards a chemical signal is called chemotaxis. The first acrasin to be discovered was cyclic adenosine monophosphate (cyclic AMP), a common cell signaling molecule, in Dictyostelium discoideum. During the aggregation phase of their life cycle, Dictyostelium discoideum amoebae communicate with each other using traveling waves of cyclic AMP. There is an amplification of cyclic AMP when they aggregate. Pre-stalk cells move toward cyclic AMP, but pre-spore cells ignore the signal. Other acrasins exist; the acrasin for Polysphondylium violaceum, purified in 1983, is the dipeptide glorin. Calcium ions too serve to attract slime mold amoebae, at least at short distances. It has been suggested that acrasins may be taxon-specific, since specificity is required to form an aggregation of genetically similar cells. Many dictyostelid species indeed do not respond to cyclic AMP, but as of 2023 their acrasins remained unknown.
Study
Use in research and teaching
The practical study of slime molds was facilitated by the introduction of the "moist culture chamber" by H. C. Gilbert and G. W. Martin in 1933. Slime molds can be used to teach convergent evolution, as the habit of forming a stalk with a sporangium that can release spores into the air, off the ground, has evolved repeatedly, such as in myxogastria (eukaryotes) and in myxobacteria (prokaryotes). Further, both the (macroscopic) dictyostelids and the (microscopic) protostelids have a phase with motile amoebae and a phase with a stalk; in the protostelids, the stalk is tiny, supporting just one spore, but the logic of airborne spore dispersal is the same.
O. R. Collins showed that the slime mold Didymium iridis had two strains (+ and −) of cells, equivalent to gametes, that these could form immortal cell lines in culture, and that the system was controlled by alleles of a single gene. This made the species a model organism for exploring incompatibility, asexual reproduction, and mating types.
Biochemicals
Slime molds have been studied for their production of unusual organic compounds, including pigments, antibiotics, and anti-cancer drugs. Pigments include naphthoquinones, physarochrome A, and compounds of tetramic acid. Bisindolylmaleimides produced by Arcyria denudata include some phosphorescent compounds. The sporophores (fruiting bodies) of Arcyria denudata are colored red by arcyriaflavins A–C, which contain an unusual indolo[2,3-a]carbazole alkaloid ring. By 2022, more than 100 pigments had been isolated from slime molds, mostly from sporophores. It has been suggested that the many yellow-to-red pigments might be useful in cosmetics. Some 42% of patients with seasonal allergic rhinitis reacted to myxogastrian spores, so the spores may contribute significantly as airborne allergens.
Computation
Slime molds share some similarities with neural systems in animals. The membranes of both slime molds and neural cells contain receptor sites, which alter electrical properties of the membrane when it is bound. Therefore, some studies on the early evolution of animal neural systems are inspired by slime molds. When a slime mold mass or mound is physically separated, the cells find their way back to re-unite. Studies on Physarum polycephalum have even shown the organism to have an ability to learn and predict periodic unfavorable conditions in laboratory experiments. John Tyler Bonner, a professor of ecology known for his studies of slime molds, argues that they are "no more than a bag of amoebae encased in a thin slime sheath, yet they manage to have various behaviors that are equal to those of animals who possess muscles and nerves with ganglia – that is, simple brains."
The slime mold algorithm is a meta-heuristic algorithm, based on the behavior of aggregated slime molds as they stream in search of food. It is described as a simple, efficient, and flexible way of solving optimization problems, such as finding the shortest path between nodes in a network. However, it can become trapped in a local optimum.
Toshiyuki Nakagaki and colleagues studied slime molds and their abilities to solve mazes by placing nodes at two points separated by a maze of plastic film. The mold explored all possible paths and solved it for the shortest path.
Traffic system inspirations

Atsushi Tero and colleagues grew Physarum in a flat wet dish, placing the mold in a central position representing Tokyo, and oat flakes surrounding it corresponding to the locations of other major cities in the Greater Tokyo Area. As Physarum avoids bright light, light was used to simulate mountains, water and other obstacles in the dish. The mold first densely filled the space with plasmodia, and then thinned the network to focus on efficiently connected branches. The network closely resembled Tokyo's rail system. P. polycephalum was used in experimental laboratory approximations of motorway networks of 14 geographical areas: Australia, Africa, Belgium, Brazil, Canada, China, Germany, Iberia, Italy, Malaysia, Mexico, the Netherlands, UK and US. The filamentary structure of P. polycephalum forming a network to food sources is similar to the large scale galaxy filament structure of the universe. This observation has led astronomers to use simulations based on the behaviour of slime molds to inform their search for dark matter.
Used as food
In central Mexico, the false puffball Enteridium lycoperdon was traditionally used as food; it was one of the species which mushroom-collectors or hongueros gathered on trips into the forest in the rainy season. One of its local names is "cheese mushroom", so called for its texture and flavor when cooked. It was salted, wrapped in a maize leaf, and baked in the ashes of a campfire; or boiled and eaten with maize tortillas. Fuligo septica was similarly collected in Mexico, cooked with onions and peppers and eaten in a tortilla. In Ecuador, Lycogala epidendrum was called "yakich" and eaten raw as an appetizer.
In popular culture
Oscar Requejo and N. Floro Andres-Rodriguez suggest that Fuligo septica may have inspired Irvin Yeaworth's 1958 film The Blob, in which a giant amoeba from space sets about engulfing people in a small American town.
See also
- Swarming motility – rapid and coordinated translocation of a bacterial population across solid or semi-solid surfaces
- Water mold – Fungus-like eukaryotic microorganism
References
- ^ Alexopoulos, Constantine J.; Mims, Charles W.; Blackwell, Meredith M. (1996). Introductory Mycology (4th ed.). New York: John Wiley and Sons. p. 776. ISBN 978-0-471-52229-4.
- ^ Panckow, Thomas (1654). Herbarium Portatile, Oder Behendes Kräuter und GewächsBuch. Berlin.
- ^ de Bary, A. (1860). "XXV.—On the Mycetozoa". Annals and Magazine of Natural History. 5 (28): 233–243. doi:10.1080/00222936008697211. ISSN 0374-5481.
- ^ Olive, Lindsay S.; Stoianovitch, Carmen (technical assistance) (1975). The Mycetozoans. Academic Press. pp. 1–7. ISBN 978-0-1252-6250-7.
- ^ Lister, Arthur; Lister, Gulielma (1911). A monograph of the Mycetozoa : a descriptive catalogue of the species in the Herbarium of the British Museum. London: Printed by order of the Trustees of the British Museum. doi:10.5962/bhl.title.21191.
- ^ Schnittler, M.; Mitchell, D. W. (2001) [2000]. "Species Diversity in Myxomycetes based on the morphological species concept – a critical examination". In Nowotny, Wolfgang; Aescht, Erna (eds.). Wolfsblut und Lohblüte – Lebensformen zwischen Tier und Pflanze [Wolves' Blood and Tan Blossom – Life forms between animals and plants]. Ausstellung im Biologiezentrum des OÖ. Landesmuseums. Vol. 73. OÖ Landes-Kultur. pp. 39–53. ISBN 978-3854740568.
- ^ Martin, G. W. (1932). "Systematic Position of the Slime Molds and Its Bearing on the Classification of the Fungi". Botanical Gazette. 93 (4): 421–335. doi:10.1086/334272. JSTOR 2471449. S2CID 84506715.
- ^ Martin, G. W. (1932). "The Systematic Position of the Myxomycetes". Mycologia. 93 (4): 119–129. doi:10.2307/3756254. JSTOR 3756254.
- ^ Copeland, H. F. (1956). The Classification of Lower Organisms. Palo Alto, California: Pacific Books.
- ^ Whittaker, R. H. (16 May 1969). "Response: Reassignment of Gymnomycota". Science. 164 (3881). American Association for the Advancement of Science (AAAS): 857. doi:10.1126/science.164.3881.857.b. ISSN 0036-8075. S2CID 239845755.
- ^ Patterson, D. J.; Sogin, M. L. (1992). "Eukaryote origins and protistan diversity". The origin and evolution of prokaryotic and eukaryotic cells. New Jersey: World Scientific. pp. 13–46. ISBN 978-9-8102-1262-9.
- ^ "Introduction to the 'Slime Molds'". University of California Museum of Paleontology.
- ^ Vallverdú, Jordi; et al. (2018). "Slime mould: the fundamental mechanisms of biological cognition". BioSystems. 165 (165): 57–70. arXiv:1712.00414. Bibcode:2018BiSys.165...57V. doi:10.1016/j.biosystems.2017.12.011. PMID 29326068. S2CID 3909678.
- ^ Baldauf, S.L.; Doolittle, W.F. (October 1997). "Origin and Evolution of the Slime Molds (Mycetozoa)". PNAS. 94 (22): 12007–120012. Bibcode:1997PNAS...9412007B. doi:10.1073/pnas.94.22.12007. PMC 23686. PMID 9342353.
- ^ Stoyneva-Gärtner, Maya; Uzunov, Blagoy; Androv, Miroslav; Ivanov, Kristian; Gärtner, Georg (21 December 2022). "Potential of Slime Molds as a Novel Source for the Cosmetics Industry". Cosmetics. 10 (1). MDPI AG: 3. doi:10.3390/cosmetics10010003. ISSN 2079-9284.
- ^ Adamatzky, Andrew (2016). Advances in Physarum Machines: Sensing and Computing with Slime Mould. Springer. p. 4. ISBN 978-3-319-26662-6.
- ^ Ples, Marek (2023-11-11). "Lab Snapshots by Marek Ples; Microbiology - The biology on a different level". weirdscience.eu. Retrieved 2023-07-02.
- ^ Ing, B. (1999). The myxomycetes of Britain and Ireland: an identification handbook. Slough, England: Richmond Publishing. pp. 4, 9. ISBN 978-0-85546-251-2.
- ^ Nannenga-Bremekamp, N.E. (1974). De Nederlandse Myxomyceten. Zuthpen: Koninklijke Nederlandse Natuurhistorische Vereniging. ISBN 978-90-03-93130-6.
- ^ Zhulidov, Daniel A.; Robarts, Richard D.; Zhulidov, Alexander V.; Zhulidova, Olga V.; Markelov, Danila A.; Rusanov, Viktor A.; Headley, John V. (2002). "Zinc accumulation by the slime mold Fuligo septica (L.) Wiggers in the former Soviet Union and North Korea". Journal of Environmental Quality. 31 (3): 1038–1042. Bibcode:2002JEnvQ..31.1038Z. doi:10.2134/jeq2002.1038. PMID 12026071.
- ^ Stephenson, Steven L. (15 June 2000). Myxomycetes. Portland: Timber Press. p. 65. ISBN 978-0-88192-439-8.
- ^ Krivomaz, Т. І.; Michaud, A.; Minter, D. W. (2012). "Metatrichia vesparium" (PDF).
- ^ Jacobson, R. (April 5, 2012). "Slime Molds: No Brains, No Feet, No Problem". PBS Newshour.
- ^ Kin, K.; Schaap, P. (March 2021). "Evolution of Multicellular Complexity in The Dictyostelid Social Amoebas". Genes. 12 (4): 487. doi:10.3390/genes12040487. PMC 8067170. PMID 33801615.
- ^ Fiore-Donno, Anna Maria; Nikolaev, Sergey I.; Nelson, Michaela; Pawlowski, Jan; Cavalier-Smith, Thomas; Baldauf, Sandra L. (January 2010). "Deep Phylogeny and Evolution of Slime Moulds (Mycetozoa)". Protist. 161 (1): 55–70. doi:10.1016/j.protis.2009.05.002. PMID 19656720.
- ^ "Species: Copromyxa arborescens M. Nesom & L. S. Olive". The Eumycetozoan Project Database. University of Arkansas. Retrieved June 26, 2010.
- ^ Brown, Matthew W.; Silberman, Jeffrey D.; Spiegel, Frederick W. (2011). "'Slime Molds' among the Tubulinea (Amoebozoa): Molecular Systematics and Taxonomy of Copromyxa". Protist. 162 (2): 277–287. doi:10.1016/j.protis.2010.09.003. ISSN 1434-4610. PMID 21112814.
- ^ Brown, Matthew W.; Silberman, Jeffrey D.; Spiegel, Frederick W. (2012). "A contemporary evaluation of the acrasids (Acrasidae, Heterolobosea, Excavata)". European Journal of Protistology. 48 (2). Elsevier BV: 103–123. doi:10.1016/j.ejop.2011.10.001. ISSN 0932-4739. PMID 22154141.
- ^ Neuhauser, Sigrid; Kirchmair, Martin; Bulman, Simon; Bass, David (2014). "Cross-kingdom host shifts of phytomyxid parasites". BMC Evolutionary Biology. 14 (1): 33. Bibcode:2014BMCEE..14...33N. doi:10.1186/1471-2148-14-33. PMC 4016497. PMID 24559266.
- ^ Tsui, Clement K. M.; Marshall, Wyth; Yokoyama, Rinka; Honda, Daiske; Lippmeier, J Casey; Craven, Kelly D.; Peterson, Paul D.; Berbee, Mary L. (January 2009). "Labyrinthulomycetes phylogeny and its implications for the evolutionary loss of chloroplasts and gain of ectoplasmic gliding". Molecular Phylogenetics and Evolution. 50 (1): 129–40. doi:10.1016/j.ympev.2008.09.027. PMID 18977305.
- ^ Deasey, Mary C.; Olive, Lindsay S. (July 1981). "Role of Golgi Apparatus in Sorogenesis by the Cellular Slime Mold Fonticula alba". Science. 213 (4507): 561–563. Bibcode:1981Sci...213..561D. doi:10.1126/science.213.4507.561. PMID 17794844.
- ^ Glimn-Lacy, Janice; Kaufman, Peter B. (2006). "Slime Molds". Botany Illustrated. Springer US. p. 45. doi:10.1007/0-387-28875-9_45. ISBN 978-0-387-28870-3.
- ^ Lindley, Lora A.; Stephenson, Steven L.; Spiegel, Frederick W. (1 July 2007). "Protostelids and myxomycetes isolated from aquatic habitats". Mycologia. 99 (4): 504–509. doi:10.3852/mycologia.99.4.504. PMID 18065001.
- ^ Hoppe, T.; Kutschera, U. (27 August 2022). "Phenotypic plasticity in plasmodial slime molds and molecular phylogeny of terrestrial vs. aquatic species". Theory in Biosciences. 141 (3). Springer: 313–319. doi:10.1007/s12064-022-00375-9. ISSN 1431-7613. PMC 9474427. PMID 36029433.
- ^ Ameen, Fuad; Almansob, Abobakr; Al-Sabri, Ahmed (2020). "Records of slime molds (Myxomycetes) from deserts and other arid areas of Saudi Arabia". Sydowia (72). Verlag Ferdinand Berger & Söhne: 171–177. doi:10.12905/0380.sydowia72-2020-0171. ISSN 0082-0598.
- ^ Glime, J. M. (2019). "Slime Molds: Ecology and Habitats – Lesser Habitats". Bryophyte Ecology. Vol. 2. Bryological Interaction. Ebook sponsored by Michigan Technological University and the International Association of Bryologists.
- ^ Spiegel, Frederick W.; Steven L. Stephenson; Harold W. Keller; Donna L Moore; James C. Cavendar (2004). "Mycetozoans". In Gregory M. Mueller; Gerald F. Bills; Mercedes S. Foster (eds.). Biodiversity of fungi: inventory and monitoring methods. New York: Elsevier Academic Press. pp. 547–576. ISBN 0125095511.
- ^ Zou, Yue; Hou, Jiangan; Guo, Songning; Li, Changtian; Li, Zhuang; Stephenson, Steven L.; Pavlov, Igor N.; Liu, Pu; Li, Yu (26 October 2022). "Diversity of Dictyostelid Cellular Slime Molds, Including Two Species New to Science, in Forest Soils of Changbai Mountain, China". Microbiology Spectrum. 10 (5). American Society for Microbiology: e0240222. doi:10.1128/spectrum.02402-22. ISSN 2165-0497. PMC 9620775. PMID 36190423.
- ^ Bonner, John Tyler (7 November 2015). "The Evolution of Evolution: Seen through the Eyes of a Slime Mold". BioScience. 65 (12). Oxford University Press: 1184–1187. doi:10.1093/biosci/biv154. ISSN 1525-3244.
- ^ Li, Yan-Da; Tihelka, Erik; Liu, Zhen-Hua; Huang, Di-Ying; Cai, Chen-Yang (23 November 2021). "New mid-Cretaceous cryptic slime mold beetles and the early evolution of Sphindidae (Coleoptera: Cucujoidea)". Arthropod Systematics & Phylogeny. 79: 587–597. doi:10.3897/asp.79.e72724. ISSN 1864-8312.
- ^ Ling, H. (2012). "Myxomycetes: Overlooked Native Plants". The Native Plant Society of New Jersey. Archived from the original on 9 June 2015. Retrieved 29 May 2018.
- ^ Chimileski, Scott; Kolter, Roberto. "Life at the Edge of Sight". www.hup.harvard.edu. Harvard University Press. Archived from the original on October 19, 2023. Retrieved 2018-01-26.
- ^ Alexopoulos, C.J. (1962). Introductory Mycology (Second ed.). New York, N.Y.: John Wiley and Sons. p. 78.
- ^ Dee, Jennifer (1960). "A Mating-type System in an Acellular Slime-mould". Nature. 185 (4715): 780–781. Bibcode:1960Natur.185..780D. doi:10.1038/185780a0. S2CID 4206149.
- ^ Moskvitch, Katia (9 July 2018). "Slime Molds Remember – but Do They Learn?". Quanta Magazine. Retrieved 2019-11-02.
- ^ Judson, Olivia (2002). Dr. Tatiana's Sex Advice To All Creation. New York: Henry Holt and Company. pp. 187–193. ISBN 978-0-8050-6332-5.
- ^ Renner, B. (2006). "Slime Mold Reproduction". BioWeb. University of Wisconsin System. Archived from the original on November 2, 2019. Retrieved 2019-11-02.
- ^ Cavender, James C.; Raper, Kenneth B. (March 1965). "The Acrasieae in Nature. Ii. Forest Soil as a Primary Habitat". American Journal of Botany. 52 (3): 297–302. doi:10.1002/j.1537-2197.1965.tb06789.x. ISSN 0002-9122.
- ^ Bonner, J. T. (2009). The Social Amoebae: The Biology of Cellular Slime Molds. Princeton University Press. ISBN 978-0-691-13939-5. JSTOR j.ctt7s6qz.
- ^ Erdos, Gregory W.; Raper, Kenneth B.; Vogen, Linda K. (June 1973). "Mating Types and Macrocyst Formation in Dictyostelium discoideum". Proceedings of the National Academy of Sciences of the United States of America. 70 (6): 1828–1830. Bibcode:1973PNAS...70.1828E. doi:10.1073/pnas.70.6.1828. PMC 433606. PMID 16592095.
- ^ Nestle, Marion; Sussman, Maurice (August 1972). "The effect of cyclic AMP on morphogernesis and enzyme accumulation in Dictyostelium discoideum". Developmental Biology. 28 (4): 545–554. doi:10.1016/0012-1606(72)90002-4. PMID 4340352.
- ^ Levine, Herbert; Reynolds, William (May 1991). "Streaming instability of aggregating slime mold amoebae". Physical Review Letters. 66 (18): 2400–2403. Bibcode:1991PhRvL..66.2400L. doi:10.1103/PhysRevLett.66.2400. PMID 10043475.
- ^ Tyson, John J.; Alexander, Kevin A.; Manoranjan, V. S.; Murray, J.D. (1989-01-01). "Spiral waves of cyclic amp in a model of slime mold aggregation". Physica D: Nonlinear Phenomena. 34 (1): 193–207. Bibcode:1989PhyD...34..193T. doi:10.1016/0167-2789(89)90234-0. ISSN 0167-2789.
- ^ Roos, W.; Nanjundiah, V.; Malchow, D.; Gerisch, G. (May 1975). "Amplification of cyclic-AMP signals in aggregating cells of Dictyostelium discoideum". FEBS Letters. 53 (2): 139–142. doi:10.1016/0014-5793(75)80005-6. PMID 166875. S2CID 29448450.
- ^ Fujimori, Taihei; Nakajima, Akihiko; Shimada, Nao; Sawai, Satoshi (March 2019). "Tissue self-organization based on collective cell migration by contact activation of locomotion and chemotaxis". Proceedings of the National Academy of Sciences of the United States of America. 116 (10): 4291–4296. Bibcode:2019PNAS..116.4291F. doi:10.1073/pnas.1815063116. PMC 6410881. PMID 30782791.
- ^ Bonner, John Tyler (1983). "Chemical Signals of Social Amoebae". Scientific American. 248 (4): 114–121. Bibcode:1983SciAm.248d.114B. doi:10.1038/scientificamerican0483-114. ISSN 0036-8733. JSTOR 24968880.
- ^ Sheikh, Sanea; Fu, Chengjie; Brown, Matthew; Baldauf, Sandra (1 March 2023), Deep origins of eukaryotic multicellularity revealed by the Acrasis kona genome and developmental transcriptomes, doi:10.21203/rs.3.rs-2587723/v1
- ^ Gilbert, H. C.; Martin, G. W. (1933). "Myxomycetes found on the bark of living trees". University of Iowa Studies in Natural History. 15: 3–8.
- ^ Keller, Harold W.; Everhart, Sydney (2010). "Importance of Myxomycetes in Biological Research and Teaching". Fungi. 3 (1 (Winter 2010)).
- ^ Steglich, W. (1 January 1989). "Slime moulds (Myxomycetes) as a source of new biologically active metabolites". Pure and Applied Chemistry. 61 (3). Walter de Gruyter GmbH: 281–288. doi:10.1351/pac198961030281. ISSN 1365-3075. S2CID 53663356.
- ^ Dembitsky, Valery M.; Řezanka, Tomáš; Spížek, Jaroslav; Hanuš, Lumír O. (2005). "Secondary metabolites of slime molds (myxomycetes)". Phytochemistry. 66 (7). Elsevier BV: 747–769. Bibcode:2005PChem..66..747D. doi:10.1016/j.phytochem.2005.02.017. ISSN 0031-9422. PMID 15797602.
- ^ Lierl, Michelle B. (2013). "Myxomycete (slime mold) spores: unrecognized aeroallergens?". Annals of Allergy, Asthma & Immunology. 111 (6). Elsevier BV: 537–541.e2. doi:10.1016/j.anai.2013.08.007. ISSN 1081-1206. PMID 24267365.
- ^ Carr, William E. S. (1989). "Chemical Signaling Systems in Lower Organisms: A Prelude to the Evolution of Chemical Communication in the Nervous System". In Anderson, Peter A.V. (ed.). Evolution of the First Nervous Systems. Boston, MA: Springer. pp. 81–94. doi:10.1007/978-1-4899-0921-3_6. ISBN 978-1-4899-0921-3.
- ^ Carr, William E. S.; Gleeson, Richard A.; Trapido-Rosenthal, Henry G. (June 1990). "The role of perireceptor events in chemosensory processes". Trends in Neurosciences. 13 (6): 212–215. doi:10.1016/0166-2236(90)90162-4. PMID 1694326. S2CID 46452914.
- ^ Lindsey, J.; Lasker, R. (1974). "Chemical Signals in the Sea: Marine Allelochemics and Evolution". Fishery Bulletin. 72 (1): 1–11.
- ^ Lenhoff, H M; Heagy, W (April 1977). "Aquatic invertebrates: model systems for study of receptor activation and evolution of receptor proteins". Annual Review of Pharmacology and Toxicology. 17 (1): 243–258. doi:10.1146/annurev.pa.17.040177.001331. PMID 17353.
- ^ Janssens, P.M.; Van Haastert, P.J. (December 1987). "Molecular basis of transmembrane signal transduction in Dictyostelium discoideum". Microbiological Reviews. 51 (4): 396–418. doi:10.1128/mr.51.4.396-418.1987. PMC 373123. PMID 2893972.
- ^ Saigusa, Tetsu; Tero, Atsushi; Nakagaki, Toshiyuki; Kuramoto, Yoshiki (January 2008). "Amoebae anticipate periodic events". Physical Review Letters. 100 (1): 018101. Bibcode:2008PhRvL.100a8101S. doi:10.1103/PhysRevLett.100.018101. hdl:2115/33004. PMID 18232821. S2CID 14710241.
- Barone, Jennifer (December 9, 2008). "#71: Slime Molds Show Surprising Degree of Intelligence". Discover. Archived from the original on December 10, 2008.
- ^ MacPherson, Kitta (January 21, 2010). "The 'sultan of slime': Biologist continues to be fascinated by organisms after nearly 70 years of study". Princeton University.
- ^ Zheng, Rong; Jia, Heming; Aualigah, Laith; Liu, Qingxin; Wang, Shuang (2021). "Deep ensemble of slime mold algorithm and arithmetic optimization algorithm for global optimization". Processes. 9 (10): 1774. doi:10.3390/pr9101774.
- ^ Nakagaki, Toshiyuki; Yamada, Hiroyasu; Toth, Agotha (September 28, 2000). "Maze-solving by an amoeboid organism". Nature. 407 (6803): 470. doi:10.1038/35035159. PMID 11028990.
- ^ Tero, A.; Takagi, S.; Saigusa, T.; et al. (January 2010). "Rules for biologically inspired adaptive network design" (PDF). Science. 327 (5964): 439–442. Bibcode:2010Sci...327..439T. doi:10.1126/science.1177894. PMID 20093467. S2CID 5001773. Archived from the original (PDF) on 2013-04-21.
- Yong, Ed (January 21, 2010). "Slime mould attacks simulates Tokyo rail network". ScienceBlogs.
- ^ Christiansen B (25 January 2010). "Slime Mold Network Engineering". Technovelgy.
- ^ Marks, P. (6 January 2010). "Designing highways the slime mould way". New Scientist.
- ^ Adamatzky, Andrew; Akl, S.; Alonso-Sanz, R.; et al. (2013). "Are motorways rational from slime mould's point of view?". International Journal of Parallel, Emergent and Distributed Systems. 28 (3): 230–248. arXiv:1203.2851. doi:10.1080/17445760.2012.685884. S2CID 15534238.
- ^ Parr, D. (18 February 2014). "Cities in motion: how slime mould can redraw our rail and road maps". The Guardian.
- ^ "Slime Mold Simulations Used to Map Dark Matter". NASA. 10 March 2020.
- ^ Wenz, J. (12 March 2020). "Slime mold helps astronomers map dark matter". Astronomy magazine.
- ^ Requejo, Oscar; Andres-Rodriguez, N. Floro (2019). "Consideraciones Etnobiologicas sobre los Mixomicetos" [Ethnobiological Considerations on Myxomycetes]. Bol. Soc. Micol. Madrid (in Spanish). 43: 25–37.





![Enteridium lycoperdon sporangium. Spores can disperse in air or water, or by slime mold flies.[21]](http://upload.wikimedia.org/wikipedia/commons/thumb/c/c2/Enteridium_lycoperdon%2C_%28Bull.%29_M.L._Farr%2C_1976_%28Reticularia_lycoperdon%29_%28cropped%29.JPG/175px-Enteridium_lycoperdon%2C_%28Bull.%29_M.L._Farr%2C_1976_%28Reticularia_lycoperdon%29_%28cropped%29.JPG)
![Metatrichia vesparium has small round sporangia that have spiral elaters to eject their lids and disperse their spores.[22]](http://upload.wikimedia.org/wikipedia/commons/thumb/1/13/Metatrichia_vesparium_82442.jpg/175px-Metatrichia_vesparium_82442.jpg)



